Cellular traction stresses increase with increasing metastatic potential
- PMID: 22389710
- PMCID: PMC3289668
- DOI: 10.1371/journal.pone.0032572
Cellular traction stresses increase with increasing metastatic potential
Abstract
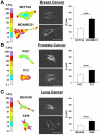
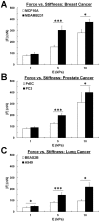
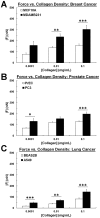
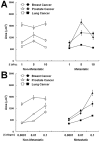
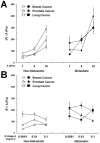
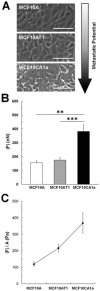
Cancer cells exist in a mechanically and chemically heterogeneous microenvironment which undergoes dynamic changes throughout neoplastic progression. During metastasis, cells from a primary tumor acquire characteristics that enable them to escape from the primary tumor and migrate through the heterogeneous stromal environment to establish secondary tumors. Despite being linked to poor prognosis, there are no direct clinical tests available to diagnose the likelihood of metastasis. Moreover, the physical mechanisms employed by metastatic cancer cells to migrate are poorly understood. Because metastasis of most solid tumors requires cells to exert force to reorganize and navigate through dense stroma, we investigated differences in cellular force generation between metastatic and non-metastatic cells. Using traction force microscopy, we found that in human metastatic breast, prostate and lung cancer cell lines, traction stresses were significantly increased compared to non-metastatic counterparts. This trend was recapitulated in the isogenic MCF10AT series of breast cancer cells. Our data also indicate that increased matrix stiffness and collagen density promote increased traction forces, and that metastatic cells generate higher forces than non-metastatic cells across all matrix properties studied. Additionally, we found that cell spreading for these cell lines has a direct relationship with collagen density, but a biphasic relationship with substrate stiffness, indicating that cell area alone does not dictate the magnitude of traction stress generation. Together, these data suggest that cellular contractile force may play an important role in metastasis, and that the physical properties of the stromal environment may regulate cellular force generation. These findings are critical for understanding the physical mechanisms of metastasis and the role of the extracellular microenvironment in metastatic progression.
Conflict of interest statement
Figures
References
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. - PubMed
-
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. - PubMed
-
- Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. - PubMed
-
- Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. - PubMed
-
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

