Secretion and N-linked glycosylation are required for prostatic acid phosphatase catalytic and antinociceptive activity
- PMID: 22389722
- PMCID: PMC3289678
- DOI: 10.1371/journal.pone.0032741
Secretion and N-linked glycosylation are required for prostatic acid phosphatase catalytic and antinociceptive activity
Abstract
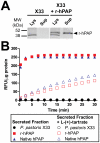
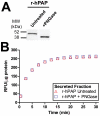
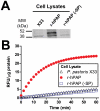
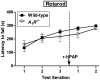
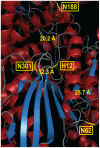
Secretory human prostatic acid phosphatase (hPAP) is glycosylated at three asparagine residues (N62, N188, N301) and has potent antinociceptive effects when administered to mice. Currently, it is unknown if these N-linked residues are required for hPAP protein stability and activity in vitro or in animal models of chronic pain. Here, we expressed wild-type hPAP and a series of Asn to Gln point mutations in the yeast Pichia pastoris X33 then analyzed protein levels and enzyme activity in cell lysates and in conditioned media. Pichia secreted wild-type recombinant (r)-hPAP into the media (6-7 mg protein/L). This protein was as active as native hPAP in biochemical assays and in mouse models of inflammatory pain and neuropathic pain. In contrast, the N62Q and N188Q single mutants and the N62Q, N188Q double mutant were expressed at lower levels and were less active than wild-type r-hPAP. The purified N62Q, N188Q double mutant protein was also 1.9 fold less active in vivo. The N301Q mutant was not expressed, suggesting a critical role for this residue in protein stability. To explicitly test the importance of secretion, a construct lacking the signal peptide of hPAP was expressed in Pichia and assayed. This "cellular" construct was not expressed at levels detectable by western blotting. Taken together, these data indicate that secretion and post-translational carbohydrate modifications are required for PAP protein stability and catalytic activity. Moreover, our findings indicate that recombinant hPAP can be produced in Pichia--a yeast strain that is used to generate biologics for therapeutic purposes.
Conflict of interest statement
Figures


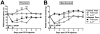
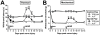
Similar articles
-
Drosophila melanogaster angiotensin I-converting enzyme expressed in Pichia pastoris resembles the C domain of the mammalian homologue and does not require glycosylation for secretion and enzymic activity.Biochem J. 1996 Aug 15;318 ( Pt 1)(Pt 1):125-31. doi: 10.1042/bj3180125. Biochem J. 1996. PMID: 8761461 Free PMC article.
-
Identification and Functional Characterization of Glycosylation of Recombinant Human Platelet-Derived Growth Factor-BB in Pichia pastoris.PLoS One. 2015 Dec 23;10(12):e0145419. doi: 10.1371/journal.pone.0145419. eCollection 2015. PLoS One. 2015. PMID: 26701617 Free PMC article.
-
Engineering of deglycosylated and plasmin resistant variants of recombinant streptokinase in Pichia pastoris.Appl Microbiol Biotechnol. 2018 Dec;102(24):10561-10577. doi: 10.1007/s00253-018-9402-x. Epub 2018 Oct 8. Appl Microbiol Biotechnol. 2018. PMID: 30298450
-
Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production.J Mol Recognit. 2005 Mar-Apr;18(2):119-38. doi: 10.1002/jmr.687. J Mol Recognit. 2005. PMID: 15565717 Review.
-
Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris.Curr Opin Biotechnol. 2002 Aug;13(4):329-32. doi: 10.1016/s0958-1669(02)00330-0. Curr Opin Biotechnol. 2002. PMID: 12323354 Review.
Cited by
-
PAPupuncture has localized and long-lasting antinociceptive effects in mouse models of acute and chronic pain.Mol Pain. 2012 Apr 23;8:28. doi: 10.1186/1744-8069-8-28. Mol Pain. 2012. PMID: 22524543 Free PMC article.
-
Emerging roles of human prostatic Acid phosphatase.Biomol Ther (Seoul). 2013 Jan;21(1):10-20. doi: 10.4062/biomolther.2012.095. Biomol Ther (Seoul). 2013. PMID: 24009853 Free PMC article. Review.
-
Optimized protocol for expression and purification of membrane-bound PglB, a bacterial oligosaccharyl transferase.Protein Expr Purif. 2013 Jun;89(2):241-50. doi: 10.1016/j.pep.2013.04.001. Epub 2013 Apr 12. Protein Expr Purif. 2013. PMID: 23583934 Free PMC article.
-
In-Depth Glycoproteomic Assay of Urinary Prostatic Acid Phosphatase.ACS Meas Sci Au. 2023 Dec 8;4(1):117-126. doi: 10.1021/acsmeasuresciau.3c00055. eCollection 2024 Feb 21. ACS Meas Sci Au. 2023. PMID: 38404489 Free PMC article.
-
Strains and Molecular Tools for Recombinant Protein Production in Pichia pastoris.Methods Mol Biol. 2022;2513:79-112. doi: 10.1007/978-1-0716-2399-2_6. Methods Mol Biol. 2022. PMID: 35781201
References
-
- Quintero IB, Araujo CL, Pulkka AE, Wirkkala RS, Herrala AM, et al. Prostatic acid phosphatase is not a prostate specific target. Cancer Res. 2007;67:6549–6554. - PubMed
-
- Ostrowski WS, Kuciel R. Human prostatic acid phosphatase: selected properties and practical applications. Clin Chim Acta. 1994;226:121–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

