Dengue virus infection-enhancing activity in serum samples with neutralizing activity as determined by using FcγR-expressing cells
- PMID: 22389741
- PMCID: PMC3289619
- DOI: 10.1371/journal.pntd.0001536
Dengue virus infection-enhancing activity in serum samples with neutralizing activity as determined by using FcγR-expressing cells
Abstract
Background: Progress in dengue vaccine development has been hampered by limited understanding of protective immunity against dengue virus infection. Conventional neutralizing antibody titration assays that use FcγR-negative cells do not consider possible infection-enhancement activity. We reasoned that as FcγR-expressing cells are the major target cells of dengue virus, neutralizing antibody titration assays using FcγR-expressing cells that determine the sum of neutralizing and infection-enhancing activity, may better reflect the biological properties of antibodies in vivo.
Methods and findings: We evaluated serum samples from 80 residents of a dengue endemic country, Malaysia, for neutralizing activity, and infection-enhancing activity at 1∶10 serum dilution by using FcγR-negative BHK cells and FcγR-expressing BHK cells. The serum samples consisted of a panel of patients with acute DENV infection (31%, 25/80) and a panel of donors without acute DENV infection (69%, 55/80). A high proportion of the tested serum samples (75%, 60/80) demonstrated DENV neutralizing activity (PRNT(50)≥10) and infection-enhancing activity. Eleven of 18 serum samples from patients with acute secondary DENV infection demonstrated neutralizing activity to the infecting serotype determined by using FcγR-negative BHK cells (PRNT(50)≥10), but not when determined by using FcγR-expressing cells.
Conclusion: Human serum samples with low neutralizing activity determined by using FcγR-negative cells showed DENV infection-enhancing activity using FcγR-expressing cells, whereas those with high neutralizing activity determined by using FcγR-negative cells demonstrate low or no infection-enhancing activity using FcγR-expressing cells. The results suggest an inverse relationship between neutralizing antibody titer and infection-enhancing activity, and that neutralizing activity determined by using FcγR-expressing cells, and not the activity determined by using FcγR-negative cells, may better reflect protection to DENV infection in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
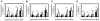

References
-
- World Health Organization. Dengue: Guidelines for diagnosis, treatment, prevention and control. 2009. whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. - PubMed
-
- Barrera R, Delgado N, Jiménez M, Valero S. Eco-epidemiological factors associated with hyperendemic dengue haemorrhagic fever in Maracay City, Venezuela. Dengue Bull. 2002;26:84–95.
-
- Lai PC, Lee SS, Kao CH, Chen YS, Huang CK, et al. Characteristics of a dengue hemorrhagic fever outbreak in 2001 in Kaohsiung. J Microbiol Immunol Infect. 2004;37:266–270. - PubMed
-
- Dengue Net-WHO Internet-based system for global surveillance of dengue fever and dengue haemorrhagic fever (dengue/DHF). http://apps.who.int/globalatlas/default.asp.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

