Glycoinositolphospholipids from Leishmania braziliensis and L. infantum: modulation of innate immune system and variations in carbohydrate structure
- PMID: 22389743
- PMCID: PMC3289616
- DOI: 10.1371/journal.pntd.0001543
Glycoinositolphospholipids from Leishmania braziliensis and L. infantum: modulation of innate immune system and variations in carbohydrate structure
Abstract
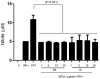
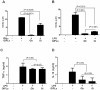
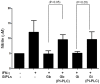
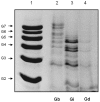
The essential role of the lipophosphoglycan (LPG) of Leishmania in innate immune response has been extensively reported. However, information about the role of the LPG-related glycoinositolphospholipids (GIPLs) is limited, especially with respect to the New World species of Leishmania. GIPLs are low molecular weight molecules covering the parasite surface and are similar to LPG in sharing a common lipid backbone and a glycan motif containing up to 7 sugars. Critical aspects of their structure and functions are still obscure in the interaction with the vertebrate host. In this study, we evaluated the role of those molecules in two medically important South American species Leishmania infantum and L. braziliensis, causative agents of visceral (VL) and cutaneous Leishmaniasis (CL), respectively. GIPLs derived from both species did not induce NO or TNF-α production by non-primed murine macrophages. Additionally, primed macrophages from mice (BALB/c, C57BL/6, TLR2-/- and TLR4-/-) exposed to GIPLs from both species, with exception to TNF-α, did not produce any of the cytokines analyzed (IL1-β, IL-2, IL-4, IL-5, IL-10, IL-12p40, IFN-γ) or p38 activation. GIPLs induced the production of TNF-α and NO by C57BL/6 mice, primarily via TLR4. Pre incubation of macrophages with GIPLs reduced significantly the amount of NO and IL-12 in the presence of IFN-γ or lipopolysaccharide (LPS), which was more pronounced with L. braziliensis GIPLs. This inhibition was reversed after PI-specific phospholipase C treatment. A structural analysis of the GIPLs showed that L. infantum has manose rich GIPLs, suggestive of type I and Hybrid GIPLs while L. braziliensis has galactose rich GIPLs, suggestive of Type II GIPLs. In conclusion, there are major differences in the structure and composition of GIPLs from L. braziliensis and L. infantum. Also, GIPLs are important inhibitory molecules during the interaction with macrophages.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




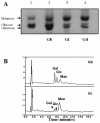
References
-
- Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. - PubMed
-
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. - PubMed
-
- Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–483. - PubMed
-
- Evans TG, Thai L, Granger DL, Hibbs JB., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993;151:907–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

