Nature-inspired total synthesis of (-)-fusarisetin A
- PMID: 22390338
- PMCID: PMC3310296
- DOI: 10.1021/ja300807e
Nature-inspired total synthesis of (-)-fusarisetin A
Abstract
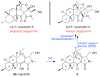
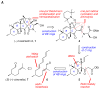
A concise, protecting group-free total synthesis of (-)-fusarisetin A (1) was efficiently achieved in nine steps from commercially available (S)-(-)-citronellal. The synthetic approach was inspired by our proposed biosynthesis of 1. Key transformations of our strategy include a facile construction of the decalin moiety that is produced via a stereoselective IMDA reaction and a one-pot TEMPO-induced radical cyclization/aminolysis that forms the C ring of 1. Our route is amenable to analogue synthesis for biological evaluation.
Figures


References
-
- Jang JH, Asami Y, Jang JP, Kim SO, Moon DO, Shin KS, Hashizume D, Muroi M, Saito T, Oh H, Kim BY, Osada H, Ahn JS. J Am Chem Soc. 2011;133:6865–6867. - PubMed
-
-
For impressive studies on (+)-migrastatin, a potent matastasis inhibitor, see: Gaul C, Njardarson JT, Danishefsky S. J J Am Chem Soc. 2003;125:6042–6043.Shan DD, Chen L, Njardarson JT, Gaul C, Ma XM, Danishefsky SJ, Huang XY. Proc Nat Acad Sci USA. 2005;102:3772–3776.Geho DH, Bandle RW, Clair T, Liotta LA. Physiology. 2005;20:194–200.
-
-
-
For equisetin isolation, characterization and biosynthesis see: Burmeister HR, Bennett GA, Vesonder RF, Hesseltine CW. Antimicrob Agents Chemother. 1974;5:634–639.Phillips NJ, Goodwin JT, Fraiman A, Cole RJ, Lynn DG. J Am Chem Soc. 1989;111:8223–8231.Sims JW, Fillmore JP, Warner DD, Schmidt EW. Chem Commun. 2005:186–188.
-
-
-
For equisetin synthesis see: Turos E, Audia JE, Danishefsky SJ. J Am Chem Soc. 1989;111:8231–8236.Burke LT, Dixon DJ, Ley SV, Rodríguez F. Org Lett. 2000;2:3611–3613.Burke LT, Dixon DJ, Ley SV, Rodríguez F. Org Biomol Chem. 2005;3:274–280.Kumiko Yuki K, Shindo M, Shishido K. Tetrahedron Lett. 2001;42:2517–2519.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

