Emerging role of ubiquitination in antiviral RIG-I signaling
- PMID: 22390971
- PMCID: PMC3294425
- DOI: 10.1128/MMBR.05012-11
Emerging role of ubiquitination in antiviral RIG-I signaling
Abstract
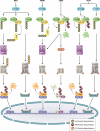
Detection of viruses by the innate immune system involves the action of specialized pattern recognition receptors. Intracellular RIG-I receptors sense the presence of viral nucleic acids in infected cells and trigger signaling pathways that lead to the production of proinflammatory and antiviral proteins. Over the past few years, posttranslational modification of RIG-I and downstream signaling proteins by different types of ubiquitination has been found to be a key event in the regulation of RIG-I-induced NF-κB and interferon regulatory factor 3 (IRF3) activation. Multiple ubiquitin ligases, deubiquitinases, and ubiquitin binding scaffold proteins contribute to both positive and negative regulation of the RIG-I-induced antiviral immune response. A better understanding of the function and activity of these proteins might eventually lead to the development of novel therapeutic approaches for management of viral diseases.
Figures
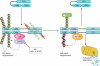


References
-
- Arimoto K, Konishi H, Shimotohno K. 2008. UbcH8 regulates ubiquitin and ISG15 conjugation to RIG-I. Mol. Immunol. 45:1078–1084 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

