The multiple signaling systems regulating virulence in Pseudomonas aeruginosa
- PMID: 22390972
- PMCID: PMC3294424
- DOI: 10.1128/MMBR.05007-11
The multiple signaling systems regulating virulence in Pseudomonas aeruginosa
Abstract
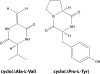
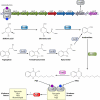
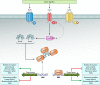
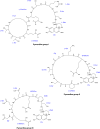
Cell-to-cell communication is a major process that allows bacteria to sense and coordinately react to the fluctuating conditions of the surrounding environment. In several pathogens, this process triggers the production of virulence factors and/or a switch in bacterial lifestyle that is a major determining factor in the outcome and severity of the infection. Understanding how bacteria control these signaling systems is crucial to the development of novel antimicrobial agents capable of reducing virulence while allowing the immune system of the host to clear bacterial infection, an approach likely to reduce the selective pressures for development of resistance. We provide here an up-to-date overview of the molecular basis and physiological implications of cell-to-cell signaling systems in Gram-negative bacteria, focusing on the well-studied bacterium Pseudomonas aeruginosa. All of the known cell-to-cell signaling systems in this bacterium are described, from the most-studied systems, i.e., N-acyl homoserine lactones (AHLs), the 4-quinolones, the global activator of antibiotic and cyanide synthesis (GAC), the cyclic di-GMP (c-di-GMP) and cyclic AMP (cAMP) systems, and the alarmones guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp), to less-well-studied signaling molecules, including diketopiperazines, fatty acids (diffusible signal factor [DSF]-like factors), pyoverdine, and pyocyanin. This overview clearly illustrates that bacterial communication is far more complex than initially thought and delivers a clear distinction between signals that are quorum sensing dependent and those relying on alternative factors for their production.
Figures









References
-
- Bainton NJ, et al. 1992. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene 116:87–91 - PubMed
-
- Balaban N, et al. 1998. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science 280:438–440 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

