Generation and exploration of new classes of antitubercular agents: The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds
- PMID: 22391032
- PMCID: PMC3304000
- DOI: 10.1016/j.bmc.2012.02.025
Generation and exploration of new classes of antitubercular agents: The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds
Abstract
Tuberculosis (TB) is a devastating disease resulting in a death every 20s. Thus, new drugs are urgently needed. Herein we report ten classes of compounds-oxazoline, oxazole, thiazoline, thiazole, pyrazole, pyridine, isoxazole, imidazo[1,2-a]pyridine, imidazo[1,2-a]pyrimidine and imidazo[1,2-c]pyrimidine-which have good (micromolar) to excellent (sub-micromolar) antitubercular potency. The 5,6-fused heteroaromatic compounds were the most potent with MIC's as low as <0.195 μM (9 and 11). Overall, the imidazo[1,2-a]pyridine class was determined to be most promising, with potency similar to isoniazid and PA-824 against replicating Mtb H(37)Rv, clinically relevant drug sensitive, multi- and extensively resistant Mtb strains as well as having good in vitro metabolic stability.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




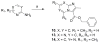
References
-
- Global tuberculosis control WHO report. 2011. WHO/HTM/TB/2011.16.
-
- Snider DE, Jr, Raviglione M, Kochi A. Global Burden of Tuberculosis, Tuberculosis: Pathogenesis, Protection, and Control. ASM Press; Washington, D.C.: 1994.
-
- Sacchettini JC, Rubin EJ, Freundlich JS. Nat Rev Microbiol. 2008;6:41–52. - PubMed
-
- Tuberculosis: A global emergency. World Health Forum. 1993;14:438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

