The cell biology of regeneration
- PMID: 22391035
- PMCID: PMC3307701
- DOI: 10.1083/jcb.201105099
The cell biology of regeneration
Abstract
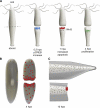
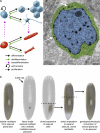
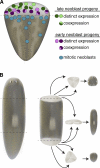
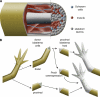
Regeneration of complex structures after injury requires dramatic changes in cellular behavior. Regenerating tissues initiate a program that includes diverse processes such as wound healing, cell death, dedifferentiation, and stem (or progenitor) cell proliferation; furthermore, newly regenerated tissues must integrate polarity and positional identity cues with preexisting body structures. Gene knockdown approaches and transgenesis-based lineage and functional analyses have been instrumental in deciphering various aspects of regenerative processes in diverse animal models for studying regeneration.
Figures
References
-
- Baguñà J., Saló E., Auladell C. 1989. Regeneration and pattern formation in planarians III. Evidence that neoblasts are totipotent stem cells and the source of blastema cells. Development. 107:77–86
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

