S6K1 determines the metabolic requirements for BCR-ABL survival
- PMID: 22391570
- PMCID: PMC3371300
- DOI: 10.1038/onc.2012.70
S6K1 determines the metabolic requirements for BCR-ABL survival
Abstract
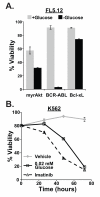
In chronic myelogenous leukemia, the constitutive activation of the BCR-ABL kinase transforms cells to an addicted state that requires glucose metabolism for survival. We investigated S6K1, a protein kinase that drives glycolysis in leukemia cells, as a target for counteracting glucose-dependent survival induced by BCR-ABL. BCR-ABL potently activated S6K1-dependent signaling and glycolysis. Although S6K1 knockdown or rapamycin treatment suppressed glycolysis in BCR-ABL-transformed cells, these treatments did not induce cell death. Instead, loss of S6K1 triggered compensatory activation of fatty-acid oxidation, a metabolic program that can support glucose-independent cell survival. Fatty-acid oxidation in response to S6K1 inactivation required the expression of the fatty-acid transporter carnitine palmitoyl transferase 1c, which was recently linked to rapamycin resistance in cancer. Finally, addition of an inhibitor of fatty-acid oxidation significantly enhanced cytotoxicity in response to S6K1 inactivation. These data indicate that S6K1 dictates the metabolic requirements mediating BCR-ABL survival and provide a rationale for combining targeted inhibitors of signal transduction, with strategies to interrupt oncogene-induced metabolism.
Figures



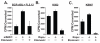
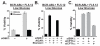

References
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–7. - PubMed
-
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL Promote Growth Factor-independent Survival through Distinct Effects on Mitochondrial Physiology. J Biol Chem. 2001;276:12041–8. - PubMed
-
- Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

