Salience network integrity predicts default mode network function after traumatic brain injury
- PMID: 22393019
- PMCID: PMC3311356
- DOI: 10.1073/pnas.1113455109
Salience network integrity predicts default mode network function after traumatic brain injury
Abstract
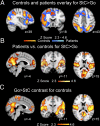
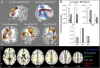
Efficient behavior involves the coordinated activity of large-scale brain networks, but the way in which these networks interact is uncertain. One theory is that the salience network (SN)--which includes the anterior cingulate cortex, presupplementary motor area, and anterior insulae--regulates dynamic changes in other networks. If this is the case, then damage to the structural connectivity of the SN should disrupt the regulation of associated networks. To investigate this hypothesis, we studied a group of 57 patients with cognitive impairments following traumatic brain injury (TBI) and 25 control subjects using the stop-signal task. The pattern of brain activity associated with stop-signal task performance was studied by using functional MRI, and the structural integrity of network connections was quantified by using diffusion tensor imaging. Efficient inhibitory control was associated with rapid deactivation within parts of the default mode network (DMN), including the precuneus and posterior cingulate cortex. TBI patients showed a failure of DMN deactivation, which was associated with an impairment of inhibitory control. TBI frequently results in traumatic axonal injury, which can disconnect brain networks by damaging white matter tracts. The abnormality of DMN function was specifically predicted by the amount of white matter damage in the SN tract connecting the right anterior insulae to the presupplementary motor area and dorsal anterior cingulate cortex. The results provide evidence that structural integrity of the SN is necessary for the efficient regulation of activity in the DMN, and that a failure of this regulation leads to inefficient cognitive control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

