How to dig deeper? Improved enrichment methods for mucin core-1 type glycopeptides
- PMID: 22393263
- PMCID: PMC3394963
- DOI: 10.1074/mcp.O111.016774
How to dig deeper? Improved enrichment methods for mucin core-1 type glycopeptides
Abstract
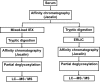
Two different workflows were tested in order to develop methods that provide deeper insight into the secreted O-glycoproteome. Bovine serum samples were subjected to lectin affinity-chromatography both at the protein- and peptide-level in order to selectively isolate glycopeptides with the most common, mucin core-1 sugar. This enrichment step was implemented with either protein-level mixed-bed ion-exchange chromatography or with peptide-level electrostatic repulsion hydrophilic interaction chromatography. Both methods led to at least 65% of the identified products being glycopeptides, in comparison to ≈ 25% without the additional chromatography steps [Darula, Z., and Medzihradszky, K. F. (2009) Affinity enrichment and characterization of mucin core-1 type glycopeptides from bovine serum. Mol. Cell. Proteomics 8, 2515-2526]. In order to improve not only the isolation but also the characterization of the glycopeptides exoglycosidases were used to eliminate carbohydrate extensions from the directly peptide-bound GalNAc units. Consequent tandem MS analysis of the mixtures using higher-energy collision-dissociation and electron-transfer dissociation led to the identification of 124 glycosylation sites in 51 proteins. While the electron-transfer dissociation data provided the bulk of the information for both modified sequence and modification site assignment, the higher-energy collision-dissociation data frequently yielded confirmation of the peptide identity, and revealed the presence of some core-2 or core-3 oligosaccharides. More than two-thirds of the sites as well as the proteins have never been reported modified.
Figures
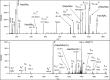
 labels the original and the charge-reduced precursor ions.
labels the original and the charge-reduced precursor ions.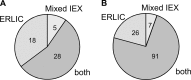

References
-
- Apweiler R., Hermjakob H., Sharon N. (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1473, 4–8 - PubMed
-
- Kenan N., Larsson A., Axelsson O., Helander A. (2011) Changes in transferrin glycosylation during pregnancy may lead to false-positive carbohydrate-deficient transferrin (CDT) results in testing for riskful alcohol consumption. Clin. Chim. Acta 412, 129–133 - PubMed
-
- Arnold J. N., Saldova R., Hamid U. M., Rudd P. M. (2008) Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Review. Proteomics 8, 3284–3293 - PubMed
-
- Halim A., Brinkmalm G., Rüetschi U., Westman-Brinkmalm A., Portelius E., Zetterberg H., Blennow K., Larson G., Nilsson J. (2011) Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc. Natl. Acad. Sci. U.S.A. 108, 11848–11853 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

