Nanobubbles for enhanced ultrasound imaging of tumors
- PMID: 22393289
- PMCID: PMC3289446
- DOI: 10.2147/IJN.S28830
Nanobubbles for enhanced ultrasound imaging of tumors
Abstract
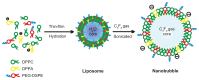
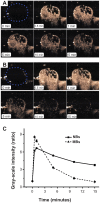
The fabrication and initial applications of nanobubbles (NBs) have shown promising results in recent years. A small particle size is a basic requirement for ultrasound contrast-enhanced agents that penetrate tumor blood vessel pores to allow for targeted imaging and therapy. However, the nanoscale size of the particles used has the disadvantage of weakening the imaging ability of clinical diagnostic ultrasound. In this work, we fabricated a lipid NBs contrast-enhanced ultrasound agent and evaluated its passive targeting ability in vivo. The results showed that the NBs were small (436.8 ± 5.7 nm), and in vitro ultrasound imaging suggested that the ultrasonic imaging ability is comparable to that of microbubbles (MBs). In vivo experiments confirmed the ability of NBs to passively target tumor tissues. The NBs remained in the tumor area for a longer period because they exhibited enhanced permeability and retention. Direct evidence was obtained by direct observation of red fluorescence-dyed NBs in tumor tissue using confocal laser scanning microscopy. We have demonstrated the ability to fabricate NBs that can be used for the in vivo contrast-enhanced imaging of tumor tissue and that have potential for drug/gene delivery.
Keywords: contrast agent; phospholipids; tumor-targeted; ultrasound.
Figures





References
-
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–818. - PubMed
-
- Skinner SA, Tutton PJ, O’Brien PE. Microvascular architecture of experimental colon tumors in the rat. Cancer Res. 1990;50(8):2411–2417. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. - PubMed
-
- Dai J, Zou S, Pei Y, Cheng D, Ai H, Shuai X. Polyethylenimine-grafted copolymer of poly(L-lysine) and poly(ethylene glycol) for gene delivery. Biomaterials. 2011;32(6):1694–1705. - PubMed
-
- Yang X, Zhu B, Dong T, Pan P, Shuai X, Inoue Y. Interactions between an anticancer drug and polymeric micelles based on biodegradable polyesters. Macromol Biosci. 2008;8(12):1116–1125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

