β-Nitro derivatives of iron corrolates
- PMID: 22394192
- PMCID: PMC3307940
- DOI: 10.1021/ic3002459
β-Nitro derivatives of iron corrolates
Abstract
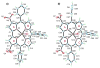
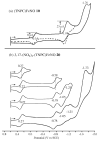
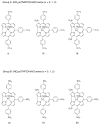
Two different methods for the regioselective nitration of different meso-triarylcorroles leading to the corresponding β-substituted nitrocorrole iron complexes have been developed. A two-step procedure affords three Fe(III) nitrosyl products-the unsubstituted corrole, the 3-nitrocorrole, and the 3,17-dinitrocorrole. In contrast, a one-pot synthetic approach drives the reaction almost exclusively to formation of the iron nitrosyl 3,17-dinitrocorrole. Electron-releasing substituents on the meso-aryl groups of the triarylcorroles induce higher yields and longer reaction times than what is observed for the synthesis of similar triarylcorroles with electron-withdrawing functionalities, and these results can be confidently attributed to the facile formation and stabilization of an intermediate iron corrole π-cation radical. Electron-withdrawing substituents on the meso-aryl groups of triarylcorrole also seem to labilize the axial nitrosyl group which, in the case of the pentafluorophenylcorrole derivative, results in the direct formation of a disubstituted iron μ-oxo dimer complex. The influence of meso-aryl substituents on the progress and products of the nitration reaction was investigated. In addition, to elucidate the most important factors which influence the redox reactivity of these different iron nitrosyl complexes, selected compounds were examined by cyclic voltammetry and thin-layer UV-visible or FTIR spectroelectrochemistry in CH(2)Cl(2).
© 2012 American Chemical Society
Figures







References
-
- Johnson AW, Kay IT. J Chem Soc. 1965:1620.
-
- Gross Z, Aviv-Harel I. Chem Eur J. 2009;15:8382–8394. - PubMed
- Kupershmidt L, Okun Z, Amit T, Mandel S, Saltsman I, Mahammed A, Bar-Am O, Gross Z, Youdim MBH. J Neurochem. 2010;113:363–373. - PubMed
- Kanamori A, Catrinescu MM, Mahammed A, Gross Z, Levin LA. J Neurochem. 2010;114:488–498. - PMC - PubMed
-
- Barbe JM, Canard G, Brandès S, Guilard R. Chem Eur J. 2007;13:2118. - PubMed
- He CL, Ren FL, Zhang XB, Han ZX. Talanta. 2006;70:364. - PubMed
- Gatto E, Malik MA, Di Natale C, Paolesse R, D’Amico A, Lundström I, Filippini D. Chem Eur J. 2008;14:6057. - PubMed
- Paolesse R, Mandoj F, Marini A, Di Natale C. In: Encyclopedia of Nanoscience and Nanotechnology. Nalwa H, editor. Valencia, CA: American Science Publishers; 2004.
- Andrioletti B, Rose E. J Chem Soc, Perkin Trans. 2002;1:715–716.
-
- Paolesse R. Synlett. 2008:2215.
-
- Gryko DT, Fox JP, Goldberg DP. J Porphyrins Phthalocyanines. 2004;8:1091.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

