Alemtuzumab as compared to alternative contemporary induction regimens
- PMID: 22394259
- PMCID: PMC3665410
- DOI: 10.1111/j.1432-2277.2012.01448.x
Alemtuzumab as compared to alternative contemporary induction regimens
Abstract
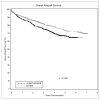
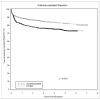
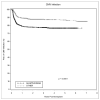
Between 1 January 2002 and 31 December 2007, our center performed 1687 adult renal transplants. A retrospective analysis was performed to compare outcomes between patients receiving alemtuzumab (n = 632) and those receiving either basiliximab (n = 690) or thymoglobulin (n = 125). Patients receiving alemtuzumab were younger (49 vs. 51 years, P = 0.02), had fewer HLA matches (1.7 vs. 2.0, P < 0.0001), were more likely to have a cytomegalovirus (CMV) donor(+)/recipient(-) transplant (22% vs. 17%, P = 0.03) and were less likely to receive a living donor allograft (32% vs. 37%, P = 0.04). Alemtuzumab recipients were less likely to receive tacrolimus (35% vs. 47%, P < 0.0001). The 1-, 3-, and 5-year cumulative incidence of antibody-mediated rejection (AMR) in alemtuzumab-treated patients was 19%, 24%, and 27%, vs. 11%, 15%, and 18% for the other group (P < 0.0001). The 1-, 3-, and 5-year allograft survival in the alemtuzumab group was 88%, 75%, and 67%, vs. 91%, 82%, and 74% for the other group (P < 0.0001). Patient survival was equivalent. Alemtuzumab was an independent risk factor for living donor allograft loss (HR 2.0, P = 0.004), opportunistic infections (HR 1.3, P = 0.01), CMV infections (HR 1.6, P = 0.001), and AMR (HR 1.5, P = 0.002). The significantly worse graft survival in the alemtuzumab cohort may be due to the increased rates of AMR and infectious complications.
© 2012 The Authors. Transplant International © 2012 European Society for Organ Transplantation.
Figures



References
-
- Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006 Sep;19(9):705–14. - PubMed
-
- Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990 Mar;35(3):118–27. - PubMed
-
- Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003 Jul 15;76(1):120–9. - PubMed
-
- Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006 Jan 15;81(1):81–7. - PubMed
-
- Knechtle SJ, Pirsch JD, H. Fechner JJ, Becker BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003 Jun;3(6):722–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

