Population-based tobacco treatment: study design of a randomized controlled trial
- PMID: 22394386
- PMCID: PMC3312843
- DOI: 10.1186/1471-2458-12-159
Population-based tobacco treatment: study design of a randomized controlled trial
Abstract
Background: Most smokers do not receive comprehensive, evidence-based treatment for tobacco use that includes intensive behavioral counseling along with pharmacotherapy. Further, the use of proven, tobacco treatments is lower among minorities than among Whites. The primary objectives of this study are to: (1) Assess the effect of a proactive care intervention (PRO) on population-level smoking abstinence rates (i.e., abstinence among all smokers including those who use and do not utilize treatment) and on utilization of tobacco treatment compared to reactive/usual care (UC) among a diverse population of smokers, (2) Compare the effect of PRO on population-level smoking abstinence rates and utilization of tobacco treatments between African American and White smokers, and (3) Determine the cost-effectiveness of the proactive care intervention.
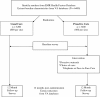
Methods/design: This prospective randomized controlled trial identifies a population-based sample of current smokers from the Department of Veterans Affairs (VA) electronic medical record health factor dataset. The proactive care intervention combines: (1) proactive outreach and (2) offer of choice of smoking cessation services (telephone or face-to-face). Proactive outreach includes mailed invitation materials followed by an outreach call that encourages smokers to seek treatment with choice of services. Proactive care participants who choose telephone care receive VA telephone counseling and access to pharmacotherapy. Proactive care participants who choose face-to-face care are referred to their VA facility's smoking cessation clinic. Usual care participants have access to standard smoking cessation services from their VA facility (e.g., pharmacotherapy, smoking cessation clinic) and from their state telephone quitline. Baseline data is collected from VA administrative databases and participant surveys. Outcomes from both groups are collected 12 months post-randomization from participant surveys and from VA administrative databases. The primary outcome is self-reported smoking abstinence, which is assessed at the population-level (i.e., among those who utilize and those who do not utilize tobacco treatment). Primary analyses will follow intention-to-treat methodology.
Discussion: This randomized trial is testing proactive outreach strategies offering choice of smoking cessation services, an innovation that if proven effective and cost-effective, will transform the way tobacco treatment is delivered. National dissemination of proactive treatment strategies could dramatically reduce tobacco-related morbidity, mortality, and health care costs.
Clinical trials registration: ClinicalTrials.gov: NCT00608426.
References
-
- Fiore MC, Croyle RT, Curry SJ, Cutler CM, Davis RM, Gordon C, Healton C, Koh HK, Orleans CT, Richling D, Satcher D, Seffrin J, Williams C, Williams LN, Keller PA, Baker TB. Preventing 3 million premature deaths and helping 5 million smokers quit: a national action plan for tobacco cessation. Am J Public Health. 2004;94:205–210. doi: 10.2105/AJPH.94.2.205. - DOI - PMC - PubMed
-
- Sherman SE, Talcot W, (Co-Chairs) Veterans Health Administration/Department of Defense Clinical Practice Guideline on Management of Tobacco Use. 2004. http://www.oqp.med.va.gov/cpg/TUC3/tuc_base.htm. Accessed 2/1/2005.
-
- McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez-Barradas MC, Sico JJ, Tindle HA, Crothers K. Validating Smoking Data From the Veteran's Affairs Health Factors Dataset, an Electronic Data Source. Nicotine Tob Res. 2011;13:1233–1239. doi: 10.1093/ntr/ntr206. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous