Akt and ERK control the proliferative response of mammary epithelial cells to the growth factors IGF-1 and EGF through the cell cycle inhibitor p57Kip2
- PMID: 22394561
- PMCID: PMC4174537
- DOI: 10.1126/scisignal.2001986
Akt and ERK control the proliferative response of mammary epithelial cells to the growth factors IGF-1 and EGF through the cell cycle inhibitor p57Kip2
Abstract
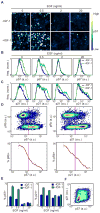
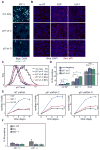
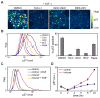
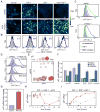
Epithelial cells respond to growth factors including epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), and insulin. Using high-content immunofluorescence microscopy, we quantitated differences in signaling networks downstream of EGF, which stimulated proliferation of mammary epithelial cells, and insulin or IGF-1, which enhanced the proliferative response to EGF but did not stimulate proliferation independently. We found that the abundance of the cyclin-dependent kinase inhibitors p21Cip1 and p57Kip2 increased in response to IGF-1 or insulin but decreased in response to EGF. Depletion of p57Kip2, but not p21Cip1, rendered IGF-1 or insulin sufficient to induce cellular proliferation in the absence of EGF. Signaling through the PI3K (phosphatidylinositol 3-kinase)-Akt-mTOR (mammalian target of rapamycin) pathway was necessary and sufficient for the increase in p57Kip2, whereas MEK [mitogen-activated or extracellular signal-regulated protein kinase (ERK) kinase]-ERK activity suppressed this increase, forming a regulatory circuit that limited proliferation in response to unaccompanied Akt activity. Knockdown of p57Kip2 enhanced the proliferative phenotype induced by tumor-associated PI3K mutant variants and released mammary epithelial acini from growth arrest during morphogenesis in three-dimensional culture. These results provide a potential explanation for the context-dependent proliferative activities of insulin and IGF-1 and for the finding that the CDKN1C locus encoding p57Kip2 is silenced in many breast cancers, which frequently show hyperactivation of the PI3K pathway. The status of p57Kip2 may thus be an important factor to assess when considering targeted therapy against the ERK or PI3K pathways.
Conflict of interest statement
Competing Interests: The authors declare no competing financial interests.
Figures

References
-
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

