Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features
- PMID: 22394600
- PMCID: PMC3382938
- DOI: 10.1182/blood-2011-09-380410
Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features
Abstract
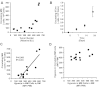
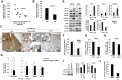
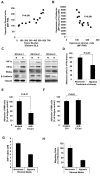
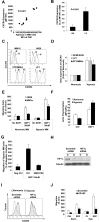
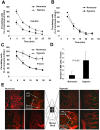
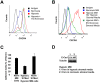
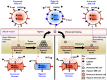
The spread of multiple myeloma (MM) involves (re)circulation into the peripheral blood and (re)entrance or homing of MM cells into new sites of the BM. Hypoxia in solid tumors was shown to promote metastasis through activation of proteins involved in the epithelial-mesenchymal transition (EMT) process. We hypothesized that MM-associated hypoxic conditions activate EMT-related proteins and promote metastasis of MM cells. In the present study, we have shown that hypoxia activates EMT-related machinery in MM cells, decreases the expression of E-cadherin, and, consequently, decreases the adhesion of MM cells to the BM and enhances egress of MM cells to the circulation. In parallel, hypoxia increased the expression of CXCR4, consequently increasing the migration and homing of circulating MM cells to new BM niches. Further studies to manipulate hypoxia to regulate tumor dissemination as a therapeutic strategy are warranted.
Figures

Comment in
-
Metastatic myeloma?Blood. 2012 Jun 14;119(24):5612-3. doi: 10.1182/blood-2012-04-417337. Blood. 2012. PMID: 22700691 No abstract available.
References
-
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. - PubMed
-
- Hideshima T, Chauhan D, Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1(7):539–544. - PubMed
-
- Pagnucco G, Cardinale G, Gervasi F. Targeting multiple myeloma cells and their bone marrow microenvironment. Ann N Y Acad Sci. 2004;1028:390–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

