Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration
- PMID: 22394620
- PMCID: PMC3446417
- DOI: 10.1186/ar3764
Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration
Abstract
Introduction: The longitudinal degradation mechanism of extracellular matrix (ECM) in the interbertebral disc remains unclear. Our objective was to elucidate catabolic and anabolic gene expression profiles and their balances in intervertebral disc degeneration using a static compression model.
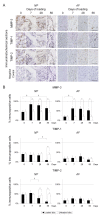
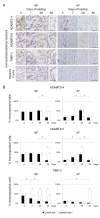
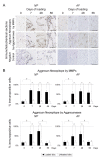
Methods: Forty-eight 12-week-old male Sprague-Dawley rat tails were instrumented with an Ilizarov-type device with springs and loaded statically at 1.3 MPa for up to 56 days. Experimental loaded and distal-unloaded control discs were harvested and analyzed by real-time reverse transcription-polymerase chain reaction (PCR) messenger RNA quantification for catabolic genes [matrix metalloproteinase (MMP)-1a, MMP-2, MMP-3, MMP-7, MMP-9, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4, and ADAMTS-5], anti-catabolic genes [tissue inhibitor of metalloproteinases (TIMP)-1, TIMP-2, and TIMP-3], ECM genes [aggrecan-1, collagen type 1-α1, and collagen type 2-α1], and pro-inflammatory cytokine genes [tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, and IL-6]. Immunohistochemistry for MMP-3, ADAMTS-4, ADAMTS-5, TIMP-1, TIMP-2, and TIMP-3 was performed to assess their protein expression level and distribution. The presence of MMP- and aggrecanase-cleaved aggrecan neoepitopes was similarly investigated to evaluate aggrecanolytic activity.
Results: Quantitative PCR demonstrated up-regulation of all MMPs and ADAMTS-4 but not ADAMTS-5. TIMP-1 and TIMP-2 were almost unchanged while TIMP-3 was down-regulated. Down-regulation of aggrecan-1 and collagen type 2-α1 and up-regulation of collagen type 1-α1 were observed. Despite TNF-α elevation, ILs developed little to no up-regulation. Immunohistochemistry showed, in the nucleus pulposus, the percentage of immunopositive cells of MMP-cleaved aggrecan neoepitope increased from 7 through 56 days with increased MMP-3 and decreased TIMP-1 and TIMP-2 immunopositivity. The percentage of immunopositive cells of aggrecanase-cleaved aggrecan neoepitope increased at 7 and 28 days only with decreased TIMP-3 immunopositivity. In the annulus fibrosus, MMP-cleaved aggrecan neoepitope presented much the same expression pattern. Aggrecanase-cleaved aggrecan neoepitope increased at 7 and 28 days only with increased ADAMTS-4 and ADAMTS-5 immunopositivity.
Conclusions: This rat tail sustained static compression model mimics ECM metabolic imbalances of MMPs, aggrecanases, and TIMPs in human degenerative discs. A dominant imbalance of MMP-3/TIMP-1 and TIMP-2 relative to ADAMTS-4 and ADAMTS-5/TIMP-3 signifies an advanced stage of intervertebral disc degeneration.
Figures



References
-
- Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443–453. - PubMed
-
- Pearce RH, Grimmer BJ. Target tissue models: the proteoglycans and degeneration of the human intervertebral disc. J Rheumatol Suppl. 1983;11:108–110. - PubMed
-
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

