Trans-differentiation of the adipose tissue-derived stem cells into neuron-like cells expressing neurotrophins by selegiline
- PMID: 22395135
- PMCID: PMC3614245
- DOI: 10.6091/ibj.1011.2012
Trans-differentiation of the adipose tissue-derived stem cells into neuron-like cells expressing neurotrophins by selegiline
Abstract
Background: Adult stem cells (ASC) are undifferentiated cells found throughout the body. These cells are promising tools for cell replacement therapy in neurodegenerative disease. Adipose tissue is the most abundant and accessible source of ASC. This study was conducted to evaluate effect of selegiline on differentiation of adipose-derived stem cells (ADSC) into functional neuron-like cells (NLC), and also level of the neurotrophin expression in differentiated cells.
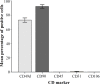
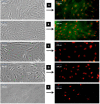
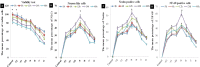
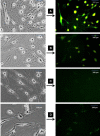
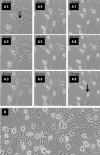
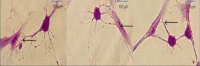
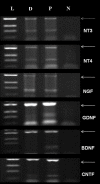
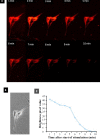
Methods: ADSC were transdifferentiated into NLC using selegiline where CD90, CD49d, CD31, CD106 and CD45 were used as markers for ADSC identification. Lipogenic and osteogenic differentiation of ADSC were used to characterize the ADSC. ADSC were treated with selegiline at different concentrations (from 10(-6) to 10(-11) mM) and time points (3, 6, 12, 24 and 48 h). Percentage of viable cells, nestin and neurofilament 68 (NF-68) immunoreactive cells were used as markers for differentiation. The optimal dose for neurotrophin expressions in differentiating cells was evaluated using reverse transcriptase-PCR. NLC function was evaluated by loading and unloading with FM1-43 dye.
Results: ADSC were immunoreactive to CD90 (95.67 ± 2.26), CD49d (71.52 ± 6.64) and CD31 (0.6 ± 0.86), but no immunoreactivity was detected for CD106 and CD45. The results of neural differentiation showed the highest percentage of nestin and NF-68 positive cells at 10(-9) mM concentration of selegiline (exposed for 24 h). The differentiated cells expressed synapsin and neurotrophin genes except brain-derived neurotrophic factor.
Conclusion: ADSC can be an alternative source in cell-based therapy for neurodegenerative diseases using selegiline to induce ADSC differentiation to neuronal lineage.
Figures



References
-
- Zhang, D.Z , Gai, L.Y , Liu, H.W , Jin, Q.H , Huang, J.H , Zhu, X.Y Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction. Chin. Med. J. (Engl). 2008;120:300–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
