TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells
- PMID: 22395485
- PMCID: PMC3336111
- DOI: 10.1105/tpc.111.094367
TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells
Abstract
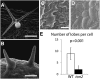
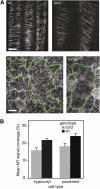
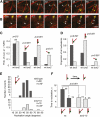
Organization of microtubules into ordered arrays involves spatial and temporal regulation of microtubule nucleation. Here, we show that acentrosomal microtubule nucleation in plant cells involves a previously unknown regulatory step that determines the geometry of microtubule nucleation. Dynamic imaging of interphase cortical microtubules revealed that the ratio of branching to in-bundle microtubule nucleation on cortical microtubules is regulated by the Arabidopsis thaliana B'' subunit of protein phosphatase 2A, which is encoded by the TONNEAU2/FASS (TON2) gene. The probability of nucleation from γ-tubulin complexes localized at the cell cortex was not affected by a loss of TON2 function, suggesting a specific role of TON2 in regulating the nucleation geometry. Both loss of TON2 function and ectopic targeting of TON2 to the plasma membrane resulted in defects in cell shape, suggesting the importance of TON2-mediated regulation of the microtubule cytoskeleton in cell morphogenesis. Loss of TON2 function also resulted in an inability for cortical arrays to reorient in response to light stimulus, suggesting an essential role for TON2 and microtubule branching nucleation in reorganization of microtubule arrays. Our data establish TON2 as a regulator of interphase microtubule nucleation and provide experimental evidence for a novel regulatory step in the process of microtubule-dependent nucleation.
Figures
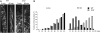



References
-
- Baulin V.A., Marques C.M., Thalmann F. (2007). Collision induced spatial organization of microtubules. Biophys. Chem. 128: 231–244 - PubMed
-
- Benschop J.J., Mohammed S., O’Flaherty M., Heck A.J., Slijper M., Menke F.L. (2007). Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6: 1198–1214 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

