Pulsing of membrane potential in individual mitochondria: a stress-induced mechanism to regulate respiratory bioenergetics in Arabidopsis
- PMID: 22395486
- PMCID: PMC3336130
- DOI: 10.1105/tpc.112.096438
Pulsing of membrane potential in individual mitochondria: a stress-induced mechanism to regulate respiratory bioenergetics in Arabidopsis
Abstract
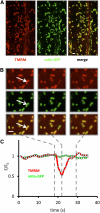
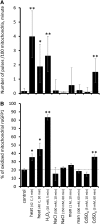
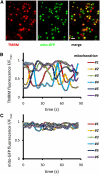
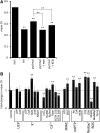
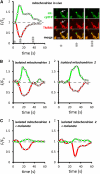
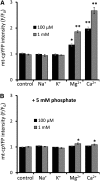
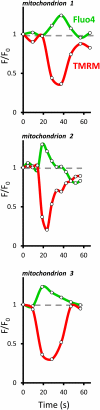
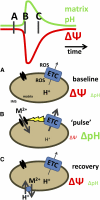
Mitochondrial ATP synthesis is driven by a membrane potential across the inner mitochondrial membrane; this potential is generated by the proton-pumping electron transport chain. A balance between proton pumping and dissipation of the proton gradient by ATP-synthase is critical to avoid formation of excessive reactive oxygen species due to overreduction of the electron transport chain. Here, we report a mechanism that regulates bioenergetic balance in individual mitochondria: a transient partial depolarization of the inner membrane. Single mitochondria in living Arabidopsis thaliana root cells undergo sporadic rapid cycles of partial dissipation and restoration of membrane potential, as observed by real-time monitoring of the fluorescence of the lipophilic cationic dye tetramethyl rhodamine methyl ester. Pulsing is induced in tissues challenged by high temperature, H(2)O(2), or cadmium. Pulses were coincident with a pronounced transient alkalinization of the matrix and are therefore not caused by uncoupling protein or by the opening of a nonspecific channel, which would lead to matrix acidification. Instead, a pulse is the result of Ca(2+) influx, which was observed coincident with pulsing; moreover, inhibitors of calcium transport reduced pulsing. We propose a role for pulsing as a transient uncoupling mechanism to counteract mitochondrial dysfunction and reactive oxygen species production.
Figures
References
-
- Aon M.A., Cortassa S., Marbán E., O’Rourke B. (2003). Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278: 44735–44744 - PubMed
-
- Balk J., Pilon M. (2011). Ancient and essential: The assembly of iron-sulfur clusters in plants. Trends Plant Sci. 16: 218–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

