Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine
- PMID: 22395605
- PMCID: PMC3316889
- DOI: 10.1038/ncomms1703
Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine
Abstract
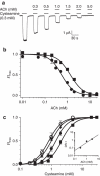
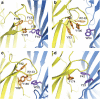
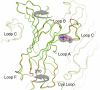
ELIC, the pentameric ligand-gated ion channel from Erwinia chrysanthemi, is a prototype for Cys-loop receptors. Here we show that acetylcholine is a competitive antagonist for ELIC. We determine the acetylcholine-ELIC cocrystal structure to a 2.9-Å resolution and find that acetylcholine binding to an aromatic cage at the subunit interface induces a significant contraction of loop C and other structural rearrangements in the extracellular domain. The side chain of the pore-lining residue F247 reorients and the pore size consequently enlarges, but the channel remains closed. We attribute the inability of acetylcholine to activate ELIC primarily to weak cation-π and electrostatic interactions in the pocket, because an acetylcholine derivative with a simple quaternary-to-tertiary ammonium substitution activates the channel. This study presents a compelling case for understanding the structural underpinning of the functional relationship between agonism and competitive antagonism in the Cys-loop receptors, providing a new framework for developing novel therapeutic drugs.
Figures

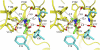

References
-
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114 (2002). - PubMed
-
- Lester H. A., Dibas M. I., Dahan D. S., Leite J. F. & Dougherty D. A. Cys-loop receptors: new twists and turns. Trends Neurosci. 27, 329–336 (2004). - PubMed
-
- Sine S. M. & Engel A. G. Recent advances in Cys-loop receptor structure and function. Nature 440, 448–455 (2006). - PubMed
-
- Taly A., Corringer P. J., Guedin D., Lestage P. & Changeux J. P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 8, 733–750 (2009). - PubMed
-
- Changeux J. P. Allosteric receptors: from electric organ to cognition. Annu. Rev. Pharmacol. Toxicol. 50, 1–38 (2010). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R37GM049202/GM/NIGMS NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- P41RR001209/RR/NCRR NIH HHS/United States
- R01GM056257/GM/NIGMS NIH HHS/United States
- P41 RR001209/RR/NCRR NIH HHS/United States
- R37 GM049202/GM/NIGMS NIH HHS/United States
- R01 GM066358/GM/NIGMS NIH HHS/United States
- R01GM066358/GM/NIGMS NIH HHS/United States
- K01DK078734/DK/NIDDK NIH HHS/United States
- R01 GM056257/GM/NIGMS NIH HHS/United States
- P30DK079307/DK/NIDDK NIH HHS/United States
- T32 GM075770/GM/NIGMS NIH HHS/United States
- T32GM075770/GM/NIGMS NIH HHS/United States
- K01 DK078734/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

