CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial
- PMID: 22395644
- PMCID: PMC3309132
- DOI: 10.1093/jnci/djs125
CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial
Abstract
Background: Adjuvant tamoxifen therapy is effective for postmenopausal women with endocrine-responsive breast cancer. Cytochrome P450 2D6 (CYP2D6) enzyme metabolizes tamoxifen to clinically active metabolites, and CYP2D6 polymorphisms may adversely affect tamoxifen efficacy. In this study, we investigated the clinical relevance of CYP2D6 polymorphisms.
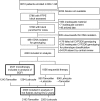
Methods: We obtained tumor tissues and isolated DNA from 4861 of 8010 postmenopausal women with hormone receptor-positive breast cancer who enrolled in the randomized, phase III double-blind Breast International Group (BIG) 1-98 trial between March 1998 and May 2003 and received tamoxifen and/or letrozole treatment. Extracted DNA was used for genotyping nine CYP2D6 single-nucleotide polymorphisms using polymerase chain reaction-based methods. Genotype combinations were used to categorize CYP2D6 metabolism phenotypes as poor, intermediate, and extensive metabolizers (PM, IM, and EM, respectively; n = 4393 patients). Associations of CYP2D6 metabolism phenotypes with breast cancer-free interval (referred to as recurrence) and treatment-induced hot flushes according to randomized endocrine treatment and previous chemotherapy were assessed. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were two-sided.
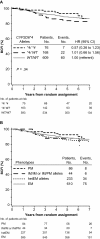
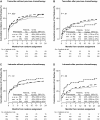
Results: No association between CYP2D6 metabolism phenotypes and breast cancer-free interval was observed among patients who received tamoxifen monotherapy without previous chemotherapy (P = .35). PM or IM phenotype had a non-statistically significantly reduced risk of breast cancer recurrence compared with EM phenotype (PM or IM vs EM, HR of recurrence = 0.86, 95% CI = 0.60 to 1.24). CYP2D6 metabolism phenotype was associated with tamoxifen-induced hot flushes (P = .020). Both PM and IM phenotypes had an increased risk of tamoxifen-induced hot flushes compared with EM phenotype (PM vs EM, HR of hot flushes = 1.24, 95% CI = 0.96 to 1.59; IM vs EM, HR of hot flushes = 1.23, 95% CI = 1.05 to 1.43).
Conclusions: CYP2D6 phenotypes of reduced enzyme activity were not associated with worse disease control but were associated with increased hot flushes, contrary to the hypothesis. The results of this study do not support using the presence or absence of hot flushes or the pharmacogenetic testing of CYP2D6 to determine whether to treat postmenopausal breast cancer patients with tamoxifen.
Trial registration: ClinicalTrials.gov NCT00004205.
Figures

Comment in
-
CYP2D6 genotype as a marker for benefit of adjuvant tamoxifen in postmenopausal women: lessons learned.J Natl Cancer Inst. 2012 Mar 21;104(6):427-8. doi: 10.1093/jnci/djs139. Epub 2012 Mar 6. J Natl Cancer Inst. 2012. PMID: 22395645 No abstract available.
-
Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial.J Natl Cancer Inst. 2012 Aug 22;104(16):1265-6; author reply 1266-8. doi: 10.1093/jnci/djs305. Epub 2012 Jul 31. J Natl Cancer Inst. 2012. PMID: 22851267 No abstract available.
-
Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial and Re: CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients.J Natl Cancer Inst. 2012 Aug 22;104(16):1263-4; author reply 1266-8. doi: 10.1093/jnci/djs312. Epub 2012 Jul 31. J Natl Cancer Inst. 2012. PMID: 22851268 No abstract available.
-
Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial.J Natl Cancer Inst. 2012 Aug 22;104(16):1264; author reply 1266-8. doi: 10.1093/jnci/djs304. Epub 2012 Jul 31. J Natl Cancer Inst. 2012. PMID: 22851270 No abstract available.
-
CYP2D6 genotype predicts tamoxifen side effects but not cancer-free or survival benefits in postmenopausal ER+ and/or PgR+ breast cancers.Pharmacogenomics. 2013 Apr;14(5):462-3. Pharmacogenomics. 2013. PMID: 23671949 No abstract available.
References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- The Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. - PubMed
-
- Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25(5):486–492. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

