Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer
- PMID: 22395661
- PMCID: PMC3314780
- DOI: 10.1038/bjc.2012.42
Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer
Abstract
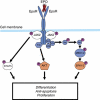
Erythropoiesis-stimulating agents (ESAs) increase red blood cell (RBC) production in bone marrow by activating the erythropoietin receptor (EpoR) on erythrocytic-progenitor cells. Erythropoiesis-stimulating agents are approved in the United States and Europe for treating anaemia in cancer patients receiving chemotherapy based on randomised, placebo-controlled trials showing that ESAs reduce RBC transfusions. Erythropoiesis-stimulating agent-safety issues include thromboembolic events and concerns regarding whether ESAs increase disease progression and/or mortality in cancer patients. Several trials have reported an association between ESA use and increased disease progression and/or mortality, whereas other trials in the same tumour types have not provided similar findings. This review thoroughly examines available evidence regarding whether ESAs affect disease progression. Both clinical-trial data on ESAs and disease progression, and preclinical data on how ESAs could affect tumour growth are summarised. Preclinical topics include (i) whether tumour cells express EpoR and could be directly stimulated to grow by ESA exposure and (ii) whether endothelial cells express EpoR and could be stimulated by ESA exposure to undergo angiogenesis and indirectly promote tumour growth. Although assessment and definition of disease progression vary across studies, the current clinical data suggest that ESAs may have little effect on disease progression in chemotherapy patients, and preclinical data indicate a direct or indirect effect of ESAs on tumour growth is not strongly supported.
Conflict of interest statement
M Aapro has received honoraria from and has had a consultant or advisory relationship with Amgen, Roche, and Sandoz. In addition, M Aapro has received research funding from Sandoz. W Jelkmann has received honoraria from and has had a consultant or advisory relationship with Amgen and Sandoz. In addition, W Jelkmann holds stock in Amgen and Roche, which are makers of Aranesp and NeoRecormon, respectively. SN Constantinescu has received honoraria from and has had a consultant or advisory relationship with Amgen. B Leyland-Jones declares no conflicts of interest.
Figures
References
-
- Aapro M, Barnadas A, Leonard RC, Marangolo M, Untch M, Ukarma L, Burger HU, Scherhag A, Osterwalder B (2009a) What is the impact of antithrombotic therapy and risk factors on the frequency of thrombovascular events in patients with metastatic breast cancer receiving epoetin beta? Eur J Cancer 45: 2984–2991 - PubMed
-
- Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, Mayordomo J, Reichert D, Pedrini JL, Ukarma L, Scherhag A, Burger HU (2008) Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol 26: 592–598 - PubMed
-
- Akan H, Guven N, Aydogdu I, Arat M, Beksac M, Dalva K (2000) Thrombopoietic cytokines in patients with iron deficiency anemia with or without thrombocytosis. Acta Haematol 103: 152–156 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

