Sirtuins as regulators of metabolism and healthspan
- PMID: 22395773
- PMCID: PMC4872805
- DOI: 10.1038/nrm3293
Sirtuins as regulators of metabolism and healthspan
Abstract
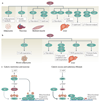
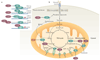
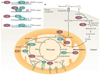
Since the beginning of the century, the mammalian sirtuin protein family (comprising SIRT1-SIRT7) has received much attention for its regulatory role, mainly in metabolism and ageing. Sirtuins act in different cellular compartments: they deacetylate histones and several transcriptional regulators in the nucleus, but also specific proteins in other cellular compartments, such as in the cytoplasm and in mitochondria. As a consequence, sirtuins regulate fat and glucose metabolism in response to physiological changes in energy levels, thereby acting as crucial regulators of the network that controls energy homeostasis and as such determines healthspan.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

