Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing
- PMID: 22395883
- PMCID: PMC3630906
- DOI: 10.1167/iovs.11-8009
Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing
Abstract
Purpose: The objective of this study was to measure the biomechanical response of the human posterior sclera in vitro and to estimate the effects of age and glaucoma.
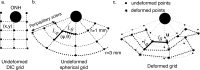
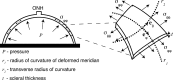
Methods: Scleral specimens from 22 donors with no history of glaucoma and 11 donors with a history of glaucoma were excised 3 mm posterior to the equator and affixed to an inflation chamber. Optic nerve cross-sections were graded to determine the presence of axon loss. The time-dependent inflation response was measured in a series of pressure-controlled load-unload tests to 30 mm Hg and creep tests to 15 and 30 mm Hg. Circumferential and meridional strains were computed from the digital image correlation displacements, and midposterior stresses were determined from pressure and deformed geometry.
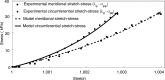
Results: Among normal specimens, older age was predictive of a stiffer response and a thinner sclera. In the age group 75 to 93, diagnosed glaucoma eyes with axon damage were thicker than normal eyes. Both damaged and undamaged glaucoma eyes had a different strain response in the peripapillary sclera characterized by a stiffer meridional response. Undamaged glaucoma eyes had slower circumferential creep rates in the peripapillary sclera than normal eyes. Glaucoma eyes were not different from normal eyes in stresses and strains in the midposterior sclera.
Conclusions: The observed differences in the biomechanical response of normal and glaucoma sclera may represent baseline properties that contribute to axon damage, or may be characteristics that result from glaucomatous disease.
Figures










References
-
- Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog Retin Eye Res. 2005;24:217–240. - PubMed
-
- Hernandez MR, Pena JDO. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997;115:389–395. - PubMed
-
- Quigley HA. Neuronal death in glaucoma. Prog in Ret and Eye Res. 1997;18:39–57. - PubMed
-
- Ethier CR. Scleral biomechanics and glaucoma—a connection? Can J Ophthalmol. 2006; 41: 9– 12, 14. - PubMed
-
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JKF, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

