The structural biology of the FGF19 subfamily
- PMID: 22396159
- PMCID: PMC3682411
- DOI: 10.1007/978-1-4614-0887-1_1
The structural biology of the FGF19 subfamily
Abstract
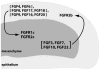
The ability of the Fibroblast Growth Factor (FGF) 19 subfamily to signal in an endocrine fashion sets this subfamily apart from the remaining five FGF subfamilies known for their paracrine functions during embryonic development. Compared to the members of paracrine FGF subfamiles, the three members of the FGF19 subfamily, namely FGF19, FGF21 and FGF23, have poor affinity for heparan sulfate (HS) and therefore can diffuse freely in the HS-rich extracellular matrix to enter into the bloodstream. In further contrast to paracrine FGFs, FGF19 subfamily members have unusually poor affinity for their cognate FGF receptors (FGFRs) and therefore cannot bind and activate them in a solely HS-dependent fashion. As a result, the FGF19 subfamily requires α/βklotho coreceptor proteins in order to bind, dimerize and activate their cognate FGFRs. This klotho-dependency also determines the tissue specificity of endocrine FGFs. Recent structural and biochemical studies have begun to shed light onto the molecular basis for the klotho-dependent endocrine mode of action of the FGF19 subfamily. Crystal structures of FGF19 and FGF23 show that the topology of the HS binding site (HBS) of FGF19 subfamily members deviates drastically from the common topology adopted by the paracrine FGFs. The distinct topologies of the HBS of FGF19 and FGF23 prevent HS from direct hydrogen bonding with the backbone atoms of the HBS of these ligands and accordingly decrease the HS binding affinity of this subfamily. Recent biochemical data reveal that the ?klotho ectodomain binds avidly to the ectodomain of FGFR1c, the main cognate FGFR of FGF23, creating a de novo high affinity binding site for the C-terminal tail of FGF23. The isolated FGF23 C-terminus can be used to effectively inhibit the formation of the FGF23-FGFR1c-αklotho complex and alleviate hypophosphatemia in renal phosphate disorders due to elevated levels of FGF23.
Figures
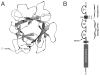
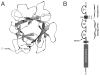







References
-
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–149. - PubMed
-
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. - PubMed
-
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16(2):107–137. - PubMed
-
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20(11):563–569. - PubMed
-
- Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237(1):18–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

