Fluorescent proteins for FRET microscopy: monitoring protein interactions in living cells
- PMID: 22396229
- PMCID: PMC3517158
- DOI: 10.1002/bies.201100098
Fluorescent proteins for FRET microscopy: monitoring protein interactions in living cells
Erratum in
- Bioessays. 2012 Jun;34(6):521
Abstract
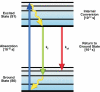
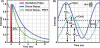
The discovery and engineering of novel fluorescent proteins (FPs) from diverse organisms is yielding fluorophores with exceptional characteristics for live-cell imaging. In particular, the development of FPs for fluorescence (or Förster) resonance energy transfer (FRET) microscopy is providing important tools for monitoring dynamic protein interactions inside living cells. The increased interest in FRET microscopy has driven the development of many different methods to measure FRET. However, the interpretation of FRET measurements is complicated by several factors including the high fluorescence background, the potential for photoconversion artifacts and the relatively low dynamic range afforded by this technique. Here, we describe the advantages and disadvantages of four methods commonly used in FRET microscopy. We then discuss the selection of FPs for the different FRET methods, identifying the most useful FP candidates for FRET microscopy. The recent success in expanding the FP color palette offers the opportunity to explore new FRET pairs.
Copyright © 2012 WILEY Periodicals, Inc.
Figures


References
-
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, et al. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229–33. - PubMed
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, et al. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–5. - PubMed
-
- Adams SR, Harootunian AT, Buechler YJ, Taylor SS, et al. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–7. - PubMed
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

