Sex differences in the brain: the not so inconvenient truth
- PMID: 22396398
- PMCID: PMC3295598
- DOI: 10.1523/JNEUROSCI.5372-11.2012
Sex differences in the brain: the not so inconvenient truth
Figures
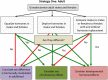
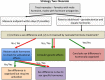
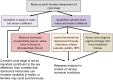
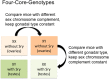
References
-
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. - PubMed
-
- Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. - PubMed
-
- Arnold AP, Wade J, Grisham W, Jacobs EC, Campagnoni AT. Sexual differentiation of the brain in songbirds. Dev Neurosci. 1996;18:124–136. - PubMed
-
- Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
