Mechanisms underlying signal filtering at a multisynapse contact
- PMID: 22396411
- PMCID: PMC6621803
- DOI: 10.1523/JNEUROSCI.5243-11.2012
Mechanisms underlying signal filtering at a multisynapse contact
Abstract
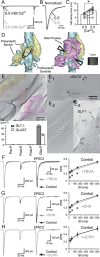
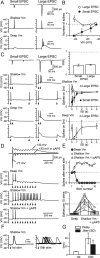
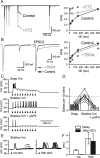
Visual information must be relayed through the lateral geniculate nucleus before it reaches the visual cortex. However, not all spikes created in the retina lead to postsynaptic spikes and properties of the retinogeniculate synapse contribute to this filtering. To understand the mechanisms underlying this filtering process, we conducted electrophysiology to assess the properties of signal transmission in the Long-Evans rat. We also performed SDS-digested freeze-fracture replica labeling to quantify the receptor and transporter distribution, as well as EM reconstruction to describe the 3D structure. To analyze the impact of transmitter diffusion on the activity of the receptors, simulations were integrated. We identified that a large contributor to the filtering is the marked paired-pulse depression at this synapse, which was intensified by the morphological characteristics of the contacts. The broad presynaptic and postsynaptic contact area restricts transmitter diffusion two dimensionally. Additionally, the presence of multiple closely arranged release sites invites intersynaptic spillover, which causes desensitization of AMPA receptors. The presence of AMPA receptors that slowly recover from desensitization along with the high presynaptic release probability and multivesicular release at each synapse also contribute to the depression. These features contrast with many other synapses where spatiotemporal spread of transmitter is limited by rapid transmitter clearance allowing synapses to operate more independently. We propose that the micrometer-order structure can ultimately affect the visual information processing.
Figures








References
-
- Blitz DM, Regehr WG. Retinogeniculate synaptic properties controlling spike number and timing in relay neurons. J Neurophysiol. 2003;90:2438–2450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
