Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial
- PMID: 22396514
- PMCID: PMC4821633
- DOI: 10.1001/jama.2012.220
Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial
Abstract
Context: Despite reported success, surgery for pharmacoresistant seizures is often seen as a last resort. Patients are typically referred for surgery after 20 years of seizures, often too late to avoid significant disability and premature death.
Objective: We sought to determine whether surgery soon after failure of 2 antiepileptic drug (AED) trials is superior to continued medical management in controlling seizures and improving quality of life (QOL).
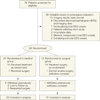
Design, setting, and participants: The Early Randomized Surgical Epilepsy Trial (ERSET) is a multicenter, controlled, parallel-group clinical trial performed at 16 US epilepsy surgery centers. The 38 participants (18 men and 20 women; aged ≥12 years) had mesial temporal lobe epilepsy (MTLE) and disabling seizues for no more than 2 consecutive years following adequate trials of 2 brand-name AEDs. Eligibility for anteromesial temporal resection (AMTR) was based on a standardized presurgical evaluation protocol. Participants were randomized to continued AED treatment or AMTR 2003-2007, and observed for 2 years. Planned enrollment was 200, but the trial was halted prematurely due to slow accrual.
Intervention: Receipt of continued AED treatment (n = 23) or a standardized AMTR plus AED treatment (n = 15). In the medical group, 7 participants underwent AMTR prior to the end of follow-up and 1 participant in the surgical group never received surgery.
Main outcome measures: The primary outcome variable was freedom from disabling seizures during year 2 of follow-up. Secondary outcome variables were health-related QOL (measured primarily by the 2-year change in the Quality of Life in Epilepsy 89 [QOLIE-89] overall T-score), cognitive function, and social adaptation.
Results: Zero of 23 participants in the medical group and 11 of 15 in the surgical group were seizure free during year 2 of follow-up (odds ratio = ∞; 95% CI, 11.8 to ∞; P < .001). In an intention-to-treat analysis, the mean improvement in QOLIE-89 overall T-score was higher in the surgical group than in the medical group but this difference was not statistically significant (12.6 vs 4.0 points; treatment effect = 8.5; 95% CI, -1.0 to 18.1; P = .08). When data obtained after surgery from participants in the medical group were excluded, the effect of surgery on QOL was significant (12.8 vs 2.8 points; treatment effect = 9.9; 95% CI, 2.2 to 17.7; P = .01). Memory decline (assessed using the Rey Auditory Verbal Learning Test) occurred in 4 participants (36%) after surgery, consistent with rates seen in the literature; but the sample was too small to permit definitive conclusions about treatment group differences in cognitive outcomes. Adverse events included a transient neurologic deficit attributed to a magnetic resonance imaging-identified postoperative stroke in a participant who had surgery and 3 cases of status epilepticus in the medical group.
Conclusions: Among patients with newly intractable disabling MTLE, resective surgery plus AED treatment resulted in a lower probability of seizures during year 2 of follow-up than continued AED treatment alone. Given the premature termination of the trial, the results should be interpreted with appropriate caution.
Trial registration: clinicaltrials.gov Identifier: NCT00040326.
Conflict of interest statement
Figures

Comment in
-
Stopping seizures early and the surgical epilepsy trial that stopped even earlier.JAMA. 2012 Mar 7;307(9):966-8. doi: 10.1001/jama.2012.251. JAMA. 2012. PMID: 22396519 No abstract available.
-
Epilepsy: Surgical therapy should not be considered a last resort for pharmacoresistant epilepsy.Nat Rev Neurol. 2012 Apr 10;8(5):238. doi: 10.1038/nrneurol.2012.55. Nat Rev Neurol. 2012. PMID: 22487752 No abstract available.
-
Treatment of refractory mesial temporal lobe epilepsy.JAMA. 2012 Jun 20;307(23):2483; author reply 2484-5. doi: 10.1001/jama.2012.4985. JAMA. 2012. PMID: 22797435 No abstract available.
-
Treatment of refractory mesial temporal lobe epilepsy.JAMA. 2012 Jun 20;307(23):2483-4; author reply 2484-5. doi: 10.1001/jama.2012.4987. JAMA. 2012. PMID: 22797436 No abstract available.
References
-
- Murray GJL, Lopez AD. Global Comparative Assessments in the Health Sector: Disease Burden, Expenditure, Intervention Packages. Geneva, Switzerland: World Health Organization; 1994.
-
- Berg AT. Understanding the delay before epilepsy surgery: who develops intractable focal epilepsy and when? CNS Spectr. 2004;9(2):136–144. - PubMed
-
- Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41(3):342–351. - PubMed
-
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40(4):445–452. - PubMed
-
- Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study: Coordination Active du Réseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia. 2001;42(4):464–475. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

