Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB
- PMID: 22396644
- PMCID: PMC3291658
- DOI: 10.1371/journal.ppat.1002555
Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB
Abstract
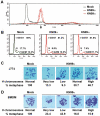
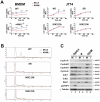
Microbial pathogens have developed efficient strategies to compromise host immune responses. Cryptococcus neoformans is a facultative intracellular pathogen, recognised as the most common cause of systemic fungal infections leading to severe meningoencephalitis, mainly in immunocompromised patients. This yeast is characterized by a polysaccharide capsule, which inhibits its phagocytosis. Whereas phagocytosis escape and macrophage intracellular survival have been intensively studied, extracellular survival of this yeast and restraint of host innate immune response are still poorly understood. In this study, we have investigated whether C. neoformans affected macrophage cell viability and whether NF-κB (nuclear factor-κB), a key regulator of cell growth, apoptosis and inflammation, was involved. Using wild-type (WT) as well as mutant strains of C. neoformans for the pathogen side, and WT and mutant cell lines with altered NF-κB activity or signalling as well as primary macrophages for the host side, we show that C. neoformans manipulated NF-κB-mediated signalling in a unique way to regulate macrophage cell fate and viability. On the one hand, serotype A strains reduced macrophage proliferation in a capsule-independent fashion. This growth decrease, which required a critical dosage of NF-κB activity, was caused by cell cycle disruption and aneuploidy, relying on fungal-induced modification of expression of several cell cycle checkpoint regulators in S and G2/M phases. On the other hand, C. neoformans infection induced macrophage apoptosis in a capsule-dependent manner with a differential requirement of the classical and alternative NF-κB signalling pathways, the latter one being essential. Together, these findings shed new light on fungal strategies to subvert host response through uncoupling of NF-κB activity in pathogen-controlled apoptosis and impairment of cell cycle progression. They also provide the first demonstration of induction of aneuploidy by a fungal pathogen, which may have wider implications for human health as aneuploidy is proposed to promote tumourigenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. - PubMed
-
- Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, et al. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–764. - PubMed
-
- Hohl TM, Rivera A, Pamer EG. Immunity to fungi. Curr Opin Immunol. 2006;18:465–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

