Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells
- PMID: 22396773
- PMCID: PMC3291570
- DOI: 10.1371/journal.pone.0032542
Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells
Abstract
Low doses of anticancer drugs have been shown to enhance antitumor immune response and increase the efficacy of immunotherapy. The molecular basis for such effects remains elusive, although selective depletion of T regulatory cells has been demonstrated. In the current studies, we demonstrate that topotecan (TPT), a topoisomerase I-targeting drug with a well-defined mechanism of action, stimulates major histocompatibility complex class I (MHC I) expression in breast cancer cells through elevated expression/secretion of interferon-β (IFN-β) and activation of type I IFN signaling. First, we show that TPT treatment elevates the expression of both total and cell-surface MHC I in breast cancer cells. Second, conditioned media from TPT-treated breast cancer ZR-75-1 cells induce elevated expression of cell-surface MHC I in drug-naïve recipient cells, suggesting the involvement of cytokines and/or other secreted molecules. Consistently, TPT-treated cells exhibit elevated expression of multiple cytokines such as IFN-β, TNF-α, IL-6 and IL-8. Third, either knocking down the type I interferon receptor subunit 1 (IFNAR1) or addition of neutralizing antibody against IFN-β results in reduced MHC I expression in TPT-treated cells. Together, these results suggest that TPT induces increased IFN-β autocrine/paracrine signaling through type I IFN receptor, resulting in the elevated MHC I expression in tumor cells. Studies have also demonstrated that other chemotherapeutic agents (e.g. etoposide, cisplatin, paclitaxel and vinblastine) similarly induce increased IFN-β secretion and elevated MHC I expression. In addition, conditioned media from γ-irradiated donor cells are shown to induce IFN-β-dependent MHC I expression in unirradiated recipient cells. In the aggregate, our results suggest that many cancer therapeutics induce elevated tumor antigen presentation through MHC I, which could represent a common mechanism for enhanced antitumor immune response through T cell cytotoxicity during metronomic chemotherapy, as well as increased efficacy of combined chemo- (or radio-)/immuno-therapy.
Conflict of interest statement
Figures
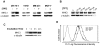
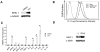



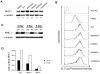
References
-
- Emens LA, Jaffee EM. Leveraging the Activity of Tumor Vaccines with Cytotoxic Chemotherapy. Cancer Research. 2005;65:8059–8064. - PubMed
-
- Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. - PubMed
-
- Machiels J-PH, Reilly RT, Emens LA, Ercolini AM, Lei RY, et al. Cyclophosphamide, Doxorubicin, and Paclitaxel Enhance the Antitumor Immune Response of Granulocyte/Macrophage-Colony Stimulating Factor-secreting Whole-Cell Vaccines in HER-2/neu Tolerized Mice. Cancer Research. 2001;61:3689–3697. - PubMed
-
- Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, et al. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30:14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

