Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury
- PMID: 22397398
- PMCID: PMC3325863
- DOI: 10.1186/1742-2094-9-48
Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury
Abstract
Background: An increase in intracellular calcium concentration [Ca2+]i is one of the first events to take place after brain ischemia. A key [Ca2+]i-regulated signaling molecule is the phosphatase calcineurin (CN), which plays important roles in the modulation of inflammatory cascades. Here, we have analyzed the role of endogenous regulator of CN 1 (Rcan1) in response to experimental ischemic stroke induced by middle cerebral artery occlusion.
Methods: Animals were subjected to focal cerebral ischemia with reperfusion. To assess the role of Rcan1 after stroke, we measured infarct volume after 48 h of reperfusion in Rcan1 knockout (KO) and wild-type (WT) mice. In vitro studies were performed in astrocyte-enriched cortical primary cultures subjected to 3% oxygen (hypoxia) and glucose deprivation (HGD). Adenoviral vectors were used to analyze the effect of overexpression of Rcan1-4 protein. Protein expression was examined by immunohistochemistry and immunoblotting and expression of mRNA by quantitative real-time Reverse-Transcription Polymerase Chain Reaction (real time qRT-PCR).
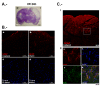
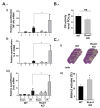
Results: Brain ischemia/reperfusion (I/R) injury in vivo increased mRNA and protein expression of the calcium-inducible Rcan1 isoform (Rcan1-4). I/R-inducible expression of Rcan1 protein occurred mainly in astroglial cells, and in an in vitro model of ischemia, HGD treatment of primary murine astrocyte cultures induced Rcan1-4 mRNA and protein expression. Exogenous Rcan1-4 overexpression inhibited production of the inflammatory marker cyclo-oxygenase 2. Mice lacking Rcan1 had higher expression of inflammation associated genes, resulting in larger infarct volumes.
Conclusions: Our results support a protective role for Rcan1 during the inflammatory response to stroke, and underline the importance of the glial compartment in the inflammatory reaction that takes place after ischemia. Improved understanding of non-neuronal mechanisms in ischemic injury promises novel approaches to the treatment of acute ischemic stroke.
Figures

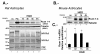
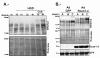

References
-
- Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510X(94)90289-5. - DOI - PubMed
-
- Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6:341–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

