5-Aza-2'-deoxycytidine induced growth inhibition of leukemia cells through modulating endogenous cholesterol biosynthesis
- PMID: 22398368
- PMCID: PMC3394957
- DOI: 10.1074/mcp.M111.016915
5-Aza-2'-deoxycytidine induced growth inhibition of leukemia cells through modulating endogenous cholesterol biosynthesis
Abstract
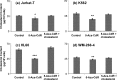
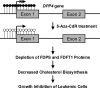
5-Aza-2'-deoxycytidine (5-Aza-CdR), a nucleoside analog that can inhibit DNA cytosine methylation, possesses potent antitumorigenic activities for myeloid disorders. Although 5-Aza-CdR is known to be incorporated into DNA and inhibit DNA (cytosine-5)-methyltransferases, the precise mechanisms underlying the drug's antineoplastic activity remain unclear. Here we utilized a mass spectrometry-based quantitative proteomic method to analyze the 5-Aza-CdR-induced perturbation of protein expression in Jurkat-T cells at the global proteome scale. Among the ≈ 2780 quantified proteins, 188 exhibited significant alteration in expression levels upon a 24-hr treatment with 5 μm 5-Aza-CdR. In particular, we found that drug treatment led to substantially reduced expression of farnesyl diphosphate synthase (FDPS) and farnesyl diphosphate farnesyltransferase (FDFT1), two important enzymes involved in de novo cholesterol synthesis. Consistent with this finding, 5-Aza-CdR treatment of leukemia (Jurkat-T, K562 and HL60) and melanoma (WM-266-4) cells led to a marked decrease in cellular cholesterol content and pronounced growth inhibition, which could be rescued by externally added cholesterol. Exposure of these cells to 5-Aza-CdR also led to epigenetic reactivation of dipeptidyl peptidase 4 (DPP4) gene. Additionally, suppression of DPP4 expression with siRNA induced elevated protein levels of FDPS and FDFT1, and increased cholesterol biosynthesis in WM-266-4 cells. Together, the results from the present study revealed, for the first time, that 5-Aza-CdR exerts its cytotoxic effects in leukemia and melanoma cells through epigenetic reactivation of DPP4 gene and the resultant inhibition of cholesterol biosynthesis in these cells.
Figures




Similar articles
-
[Growth and gene expression of leukemia cell after treated with methylation inhibitor 5-aza-2'-deoxycytidine].Zhonghua Xue Ye Xue Za Zhi. 2004 Aug;25(8):486-90. Zhonghua Xue Ye Xue Za Zhi. 2004. PMID: 15555266 Chinese.
-
Pharmacological approach for optimization of the dose schedule of 5-Aza-2'-deoxycytidine (Decitabine) for the therapy of leukemia.Leukemia. 1997 Feb;11(2):175-80. doi: 10.1038/sj.leu.2400550. Leukemia. 1997. PMID: 9009076 Review.
-
Enhancement of antineoplastic action of 5-aza-2'-deoxycytidine by zebularine on L1210 leukemia.Anticancer Drugs. 2005 Mar;16(3):301-8. doi: 10.1097/00001813-200503000-00009. Anticancer Drugs. 2005. PMID: 15711182
-
Pharmacological approach for optimization of the dose schedule of 5-Aza-2'-deoxycytidine (Decitabine) for the therapy of leukemia.Leukemia. 1997 Mar;11 Suppl 1:S1-6. Leukemia. 1997. PMID: 9130684 Review.
-
[Effect of 5-aza-2'-deoxycytidine on growth and methylation of RUNX3 gene in human pancreatic cancer cell line MiaPaca2].Zhonghua Zhong Liu Za Zhi. 2013 Jan;35(1):17-21. doi: 10.3760/cma.j.issn.0253-3766.2013.01.004. Zhonghua Zhong Liu Za Zhi. 2013. PMID: 23648294 Chinese.
Cited by
-
Quantitative proteomic analysis revealed N'-nitrosonornicotine-induced down-regulation of nonmuscle myosin II and reduced cell migration in cultured human skin fibroblast cells.J Proteome Res. 2013 Mar 1;12(3):1282-8. doi: 10.1021/pr3009397. Epub 2013 Jan 28. J Proteome Res. 2013. PMID: 23305604 Free PMC article.
-
Cd²⁺-induced alteration of the global proteome of human skin fibroblast cells.J Proteome Res. 2014 Mar 7;13(3):1677-87. doi: 10.1021/pr401159f. Epub 2014 Feb 21. J Proteome Res. 2014. PMID: 24527689 Free PMC article.
-
Five-aza-2'-deoxycytidine-induced hypomethylation of cholesterol 25-hydroxylase gene is responsible for cell death of myelodysplasia/leukemia cells.Sci Rep. 2015 Nov 18;5:16709. doi: 10.1038/srep16709. Sci Rep. 2015. PMID: 26577244 Free PMC article.
-
The role of cholesterol metabolism in leukemia.Blood Sci. 2019 Sep 17;1(1):44-49. doi: 10.1097/BS9.0000000000000016. eCollection 2019 Aug. Blood Sci. 2019. PMID: 35402792 Free PMC article. Review.
-
Comparative Proteomic Study of Fatty Acid-treated Myoblasts Reveals Role of Cox-2 in Palmitate-induced Insulin Resistance.Sci Rep. 2016 Feb 22;6:21454. doi: 10.1038/srep21454. Sci Rep. 2016. PMID: 26899878 Free PMC article.
References
-
- Holliday R. (1987) The inheritance of epigenetic defects. Science 238, 163–170 - PubMed
-
- Jones P. A., Baylin S. B. (2002) The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3, 415–428 - PubMed
-
- Yoo C. B., Jones P. A. (2006) Epigenetic therapy of cancer: past, present and future. Nat. Rev. Drug Discov. 5, 37–50 - PubMed
-
- Jones P. A., Taylor S. M. (1980) Cellular-differentiation, cytidine analogs and DNA methylation. Cell 20, 85–93 - PubMed
-
- Christman J. K. (2002) 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21, 5483–5495 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

