Phase transitions in the assembly of multivalent signalling proteins
- PMID: 22398450
- PMCID: PMC3343696
- DOI: 10.1038/nature10879
Phase transitions in the assembly of multivalent signalling proteins
Abstract
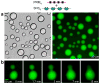
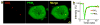
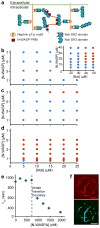
Cells are organized on length scales ranging from ångström to micrometres. However, the mechanisms by which ångström-scale molecular properties are translated to micrometre-scale macroscopic properties are not well understood. Here we show that interactions between diverse synthetic, multivalent macromolecules (including multi-domain proteins and RNA) produce sharp liquid-liquid-demixing phase separations, generating micrometre-sized liquid droplets in aqueous solution. This macroscopic transition corresponds to a molecular transition between small complexes and large, dynamic supramolecular polymers. The concentrations needed for phase transition are directly related to the valency of the interacting species. In the case of the actin-regulatory protein called neural Wiskott-Aldrich syndrome protein (N-WASP) interacting with its established biological partners NCK and phosphorylated nephrin, the phase transition corresponds to a sharp increase in activity towards an actin nucleation factor, the Arp2/3 complex. The transition is governed by the degree of phosphorylation of nephrin, explaining how this property of the system can be controlled to regulatory effect by kinases. The widespread occurrence of multivalent systems suggests that phase transitions may be used to spatially organize and biochemically regulate information throughout biology.
© 2012 Macmillan Publishers Limited. All rights reserved
Figures

References
-
- Jones N, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. - PubMed
-
- Flory PJ. Principles of Polymer Chemistry. Cornell University Press; 1953.
-
- Cohen RJ, Benedek GB. Equilibrium and Kinetic Theory of Polymerization and the Sol-Gel Transition. J Phys Chem. 1982;86:3696–3714.
-
- Lehn JM. Supramolecular Polymer Chemistry--Scope and Perspectives. Polym Int. 2002;51:825–839.
-
- Tanaka F. Polymer Physics: Applications to Molecular Association and Thermoreversible Gelation. Cambridge University Press; 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

