Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation
- PMID: 22399264
- PMCID: PMC3610325
- DOI: 10.1126/scitranslmed.3003509
Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation
Abstract
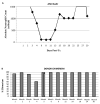
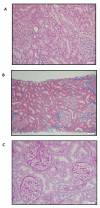
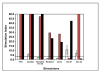
The toxicity of chronic immunosuppressive agents required for organ transplant maintenance has prompted investigators to pursue approaches to induce immune tolerance. We developed an approach using a bioengineered mobilized cellular product enriched for hematopoietic stem cells (HSCs) and tolerogenic graft facilitating cells (FCs) combined with nonmyeloablative conditioning; this approach resulted in engraftment, durable chimerism, and tolerance induction in recipients with highly mismatched related and unrelated donors. Eight recipients of human leukocyte antigen (HLA)-mismatched kidney and FC/HSC transplants underwent conditioning with fludarabine, 200-centigray total body irradiation, and cyclophosphamide followed by posttransplant immunosuppression with tacrolimus and mycophenolate mofetil. Subjects ranged in age from 29 to 56 years. HLA match ranged from five of six loci with related donors to one of six loci with unrelated donors. The absolute neutrophil counts reached a nadir about 1 week after transplant, with recovery by 2 weeks. Multilineage chimerism at 1 month ranged from 6 to 100%. The conditioning was well tolerated, with outpatient management after postoperative day 2. Two subjects exhibited transient chimerism and were maintained on low-dose tacrolimus monotherapy. One subject developed viral sepsis 2 months after transplant and experienced renal artery thrombosis. Five subjects experienced durable chimerism, demonstrated immunocompetence and donor-specific tolerance by in vitro proliferative assays, and were successfully weaned off all immunosuppression 1 year after transplant. None of the recipients produced anti-donor antibody or exhibited engraftment syndrome or graft-versus-host disease. These results suggest that manipulation of a mobilized stem cell graft and nonmyeloablative conditioning represents a safe, practical, and reproducible means of inducing durable chimerism and donor-specific tolerance in solid organ transplant recipients.
Figures



Comment in
-
The quest for transplantation tolerance: have we finally sipped from the cup?Sci Transl Med. 2012 Mar 7;4(124):124fs5. doi: 10.1126/scitranslmed.3003678. Sci Transl Med. 2012. PMID: 22399262 Free PMC article.
-
Cooking up tolerance: has a new recipe been created?Am J Transplant. 2012 Jul;12(7):1667-9. doi: 10.1111/j.1600-6143.2012.04154.x. Epub 2012 Jun 15. Am J Transplant. 2012. PMID: 22702487 Free PMC article. No abstract available.
-
The candle illuminating the pathway to tolerance?Am J Kidney Dis. 2012 Oct;60(4):521-3. doi: 10.1053/j.ajkd.2012.06.009. Epub 2012 Jul 17. Am J Kidney Dis. 2012. PMID: 22809762 No abstract available.
References
-
- Ildstad ST, Shirwan H, Leventhal J. Is durable macrochimerism key to achieving clinical transplantation tolerance? Curr. Opin. Organ Transplant. 2011;16:343–344. - PubMed
-
- Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. - PubMed
-
- Scandling JD, Busque S, jbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008;358:362–368. - PubMed
-
- Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman HA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr., Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

