Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial
- PMID: 22399690
- PMCID: PMC3329632
- DOI: 10.1136/bjophthalmol-2011-300726
Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial
Abstract
Background/aims: To evaluate the cost-effectiveness of ranibizumab as either monotherapy or combined with laser therapy, compared with laser monotherapy, in the treatment of diabetic macular oedema (DME) causing visual impairment from a UK healthcare payer perspective.
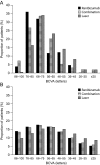
Methods: A Markov model simulated long-term outcomes and costs of treating DME in one eye (BCVA ≤75 letters) based on data from the RESTORE Phase III trial. Outcomes measured in quality-adjusted life-years (QALYs) were simulated for a 15-year time horizon based on 12-month follow-up from RESTORE and published long-term data. Costs included treatment, disease monitoring, visual impairment and blindness (at 2010 price levels).
Results: Ranibizumab monotherapy resulted in a 0.17 QALY gain at an incremental cost of £4191 relative to laser monotherapy, yielding an incremental cost-effectiveness ratio (ICER) of £24 028. Probabilistic sensitivity analysis showed a 64% probability of being cost-effective at a threshold of £30 000 per QALY. Combined ranibizumab and laser therapy resulted in a 0.13 QALY gain at an incremental cost of £4695 relative to laser monotherapy (ICER £36 106; 42% probability of ICER <£30 000).
Conclusions: Based on RESTORE 1-year follow-up data, ranibizumab monotherapy appears to be cost-effective relative to laser monotherapy, the current standard of care. Cost-effectiveness of combination therapy is less certain. Ongoing studies will further inform on disease progression and the need for additional ranibizumab treatment.
Conflict of interest statement
Figures
References
-
- Royal College of Ophthalmologists Guidelines for Diabetic Retinopathy. London, UK: Royal College of Ophthalmologists, 2005
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–36 - PubMed
-
- Minassian DC, Owens DR, Reidy A. Prevalence of diabetic macular oedema and related health and social care resource use in England. Br J Ophthalmol. Published Online First: 20 May 2011. doi:10.1136/bjo.2011.204040 - DOI - PubMed
-
- Lanzetta P, van Nuys K, Tran I, et al. The Economic Burden of Diabetic Macular Edema from a U.S. Private Payer Perspective. Fort Lauderdale, FL, USA: Association for Research in Vision and Ophthalmology (ARVO), Poster A370, 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
