Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo
- PMID: 22399752
- PMCID: PMC3548448
- DOI: 10.1523/JNEUROSCI.5368-11.2012
Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo
Abstract
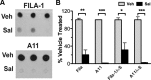
In Parkinson's disease (PD) and other α-synucleinopathies, prefibrillar α-synuclein (αS) oligomer is implicated in the pathogenesis. However, toxic αS oligomers observed using in vitro systems are not generally seen to be associated with α-synucleinopathy in vivo. Thus, the pathologic significance of αS oligomers to αS neurotoxicity is unknown. Herein, we show that, αS that accumulate within endoplasmic reticulum (ER)/microsome forms toxic oligomers in mouse and human brain with the α-synucleinopathy. In the mouse model of α-synucleinopathy, αS oligomers initially form before the onset of disease and continue to accumulate with the disease progression. Significantly, treatment of αS transgenic mice with Salubrinal, an anti-ER stress compound that delays the onset of disease, reduces ER accumulation of αS oligomers. These results indicate that αS oligomers with toxic conformation accumulate in ER, and αS oligomer-dependent ER stress is pathologically relevant for PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

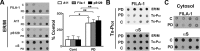
Comment in
-
The stress of misfolding.Nat Rev Neurosci. 2012 Apr 4;13(5):290. doi: 10.1038/nrn3235. Nat Rev Neurosci. 2012. PMID: 22473482 No abstract available.
-
Neurodegenerative disease: The stress of misfolding.Nat Rev Drug Discov. 2012 Apr 30;11(5):352-3. doi: 10.1038/nrd3731. Nat Rev Drug Discov. 2012. PMID: 22543462 No abstract available.
References
-
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. - PubMed
-
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. - PMC - PubMed
-
- Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc. 2006;1:1872–1878. - PubMed
-
- Croze EM, Morré DJ. Isolation of plasma membrane, golgi apparatus, and endoplasmic reticulum fractions from single homogenates of mouse liver. J Cell Physiol. 1984;119:46–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS055776/NS/NINDS NIH HHS/United States
- NS038065/NS/NINDS NIH HHS/United States
- R01 NS076160/NS/NINDS NIH HHS/United States
- R01 ES017384/ES/NIEHS NIH HHS/United States
- R01 NS038065/NS/NINDS NIH HHS/United States
- R01 AG029401/AG/NIA NIH HHS/United States
- P01 NS038065/NS/NINDS NIH HHS/United States
- R56 NS038065/NS/NINDS NIH HHS/United States
- P50 NS038377/NS/NINDS NIH HHS/United States
- ES017384/ES/NIEHS NIH HHS/United States
- R21 NS049088/NS/NINDS NIH HHS/United States
- R21 NS055776/NS/NINDS NIH HHS/United States
- NS0380377/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
