The microtubule poison vinorelbine kills cells independently of mitotic arrest and targets cells lacking the APC tumour suppressor more effectively
- PMID: 22399804
- PMCID: PMC3311929
- DOI: 10.1242/jcs.091843
The microtubule poison vinorelbine kills cells independently of mitotic arrest and targets cells lacking the APC tumour suppressor more effectively
Abstract
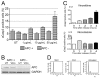
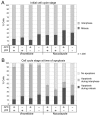
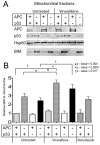
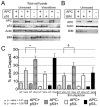
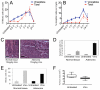
Colorectal cancers commonly carry truncation mutations in the adenomatous polyposis coli (APC) gene. The APC protein contributes to the stabilization of microtubules. Consistently, microtubules in cells lacking APC depolymerize more readily in response to microtubule-destabilizing drugs. This raises the possibility that such agents are suitable for treatment of APC-deficient cancers. However, APC-deficient cells have a compromised spindle assembly checkpoint, which renders them less sensitive to killing by microtubule poisons whose toxicity relies on the induction of prolonged mitotic arrest. Here, we describe the novel discovery that the clinically used microtubule-depolymerizing drug vinorelbine (Navelbine) kills APC-deficient cells in culture and in intestinal tissue more effectively than it kills wild-type cells. This is due to the ability of vinorelbine to kill cells in interphase independently of mitotic arrest. Consistent with a role for p53 in cell death in interphase, depletion of p53 renders cells less sensitive to vinorelbine, but only in the presence of wild-type APC. The pro-apoptotic protein BIM (also known as BCL2L11) is recruited to mitochondria in response to vinorelbine, where it can inhibit the anti-apoptotic protein BCL2, suggesting that BIM mediates vinorelbine-induced cell death. This recruitment of BIM is enhanced in cells lacking APC. Consistently, BIM depletion dampens the selective effect of vinorelbine on these cells. Our findings reveal that vinorelbine is a potential therapeutic agent for colorectal cancer, but they also illustrate the importance of the APC tumour suppressor status when predicting therapeutic efficacy.
Figures
References
-
- Beswick R. W., Ambrose H. E., Wagner S. D. (2006). Nocodazole, a microtubule depolymerising agent, induces apoptosis of chronic lymphocytic leukaemia cells associated with changes in Bcl-2 phosphorylation and expression. Leuk. Res. 30, 427-436 - PubMed
-
- Bourgarel-Rey V., Savry A., Hua G., Carré M., Bressin C., Chacon C., Imbert J., Braguer D., Barra Y. (2009). Transcriptional down-regulation of Bcl-2 by vinorelbine: identification of a novel binding site of p53 on Bcl-2 promoter. Biochem. Pharmacol. 78, 1148-1156 - PubMed
-
- Brocardo M., Lei Y., Tighe A., Taylor S. S., Mok M. T. S., Henderson B. R. (2008). Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: regulation of Bcl-2 and implications for cell survival. J. Biol. Chem. 283, 5950-5959 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

