Adenylyl cyclase AC8 directly controls its micro-environment by recruiting the actin cytoskeleton in a cholesterol-rich milieu
- PMID: 22399809
- PMCID: PMC3311928
- DOI: 10.1242/jcs.091090
Adenylyl cyclase AC8 directly controls its micro-environment by recruiting the actin cytoskeleton in a cholesterol-rich milieu
Abstract
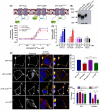
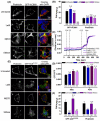
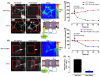
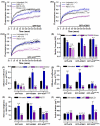
The central and pervasive influence of cAMP on cellular functions underscores the value of stringent control of the organization of adenylyl cyclases (ACs) in the plasma membrane. Biochemical data suggest that ACs reside in membrane rafts and could compartmentalize intermediary scaffolding proteins and associated regulatory elements. However, little is known about the organization or regulation of the dynamic behaviour of ACs in a cellular context. The present study examines these issues, using confocal image analysis of various AC8 constructs, combined with fluorescence recovery after photobleaching and fluorescence correlation spectroscopy. These studies reveal that AC8, through its N-terminus, enhances the cortical actin signal at the plasma membrane; an interaction that was confirmed by GST pull-down and immunoprecipitation experiments. AC8 also associates dynamically with lipid rafts; the direct association of AC8 with sterols was confirmed in Förster resonance energy transfer experiments. Disruption of the actin cytoskeleton and lipid rafts indicates that AC8 tracks along the cytoskeleton in a cholesterol-enriched domain, and the cAMP that it produces contributes to sculpting the actin cytoskeleton. Thus, an adenylyl cyclase is shown not just to act as a scaffold, but also to actively orchestrate its own micro-environment, by associating with the cytoskeleton and controlling the association by producing cAMP, to yield a highly organized signalling hub.
Figures





References
-
- Bacskai B. J., Hochner B., Mahaut-Smith M., Adams S. R., Kaang B. K., Kandel E. R., Tsien R. Y. (1993). Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science 260, 222-226 - PubMed
-
- Boyajian C. L., Garritsen A., Cooper D. M. F. (1991). Bradykinin stimulates Ca2+ mobilization in NCB-20 cells leading to direct inhibition of adenylylcyclase. A novel mechanism for inhibition of cAMP production. J. Biol. Chem. 266, 4995-5003 - PubMed
-
- Brown D. A., London E. (1998). Functions of lipid rafts in biological membranes. Ann. Rev. Cell Dev. Biol. 14, 111-136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

