Challenges for heart disease stem cell therapy
- PMID: 22399855
- PMCID: PMC3295632
- DOI: 10.2147/VHRM.S25665
Challenges for heart disease stem cell therapy
Abstract
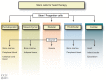
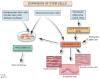
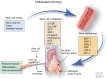
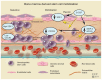
Cardiovascular diseases (CVDs) are the leading cause of death worldwide. The use of stem cells to improve recovery of the injured heart after myocardial infarction (MI) is an important emerging therapeutic strategy. However, recent reviews of clinical trials of stem cell therapy for MI and ischemic heart disease recovery report that less than half of the trials found only small improvements in cardiac function. In clinical trials, bone marrow, peripheral blood, or umbilical cord blood cells were used as the source of stem cells delivered by intracoronary infusion. Some trials administered only a stem cell mobilizing agent that recruits endogenous sources of stem cells. Important challenges to improve the effectiveness of stem cell therapy for CVD include: (1) improved identification, recruitment, and expansion of autologous stem cells; (2) identification of mobilizing and homing agents that increase recruitment; and (3) development of strategies to improve stem cell survival and engraftment of both endogenous and exogenous sources of stem cells. This review is an overview of stem cell therapy for CVD and discusses the challenges these three areas present for maximum optimization of the efficacy of stem cell therapy for heart disease, and new strategies in progress.
Keywords: engraftment; expansion; homing; mobilization; survival.
Figures
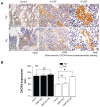
References
-
- George JC. Stem cell therapy in acute myocardial infarction: a review of clinical trials. Trans Res. 2010;155(1):10–19. - PubMed
-
- Mozid AM, Arnous S, Sammut EC, Mathur A. Stem cell therapy for heart diseases. Br Med Bull. 2011;98:143–159. - PubMed
-
- Wen Y, Meng L, Xie J, Ouyang J. Direct autologous bone marrow-derived stem cell transplantation for ischemic heart disease: a meta-analysis. Expert Opin Biol Ther. 2011;11(5):559–567. - PubMed
-
- Hirsch A, Nijveldt R, van der Vleuten PA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2011;32(14):1736–1747. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

