Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion
- PMID: 22400029
- PMCID: PMC3287024
- DOI: 10.1155/2012/142530
Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion
Abstract
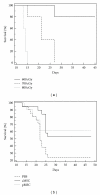
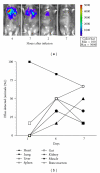
Marrow stromal cells (MSCs, also termed mesenchymal stem cells) have been proposed as a promising cellular therapy for tissue injury including radiation-induced marrow failure, but evidence for a direct effect is lacking. To assess the effects of MSCs on survival after lethal irradiation, we infused syngeneic MSCs (either as immortalized MSCs clones or primary MSCs) intravenously into wild-type C57/Bl6 mice within 24 hours of lethal total body irradiation (TBI). Mice receiving either of the MSC preparations had significantly improved survival when compared to controls. In vivo imaging, immune histochemistry, and RT-PCR employed to detect MSCs indicated that the infused MSCs were predominantly localized to the lungs and rapidly cleared following infusion. Our results suggest that a single infusion of MSCs can improve survival after otherwise lethal TBI but the effect is not due to a direct interaction with, or contribution to, the damaged marrow by MSCs.
Figures
References
-
- Karkanitsa LV. Radiation damage to hematopoiesis: what do we know better? Stem Cells. 1997;15:71–73. - PubMed
-
- Hérodin F, Grenier N, Drouet M. Revisiting therapeutic strategies in radiation casualties. Experimental Hematology. 2007;35(4, supplement 1):28–33. - PubMed
-
- Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. International Journal of Radiation Biology. 2004;80(1):3–10. - PubMed
-
- Hérodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Experimental Hematology. 2005;33(10):1071–1080. - PubMed
-
- Gale RP, Butturini A. The role of hematopoietic growth factors in nuclear and radiation accidents. Experimental Hematology. 1990;18(8):958–964. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

