Inactivation of the budded virus of Autographa californica M nucleopolyhedrovirus by gloverin
- PMID: 22401766
- PMCID: PMC3327827
- DOI: 10.1016/j.jip.2012.02.007
Inactivation of the budded virus of Autographa californica M nucleopolyhedrovirus by gloverin
Abstract
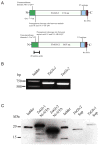
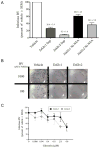
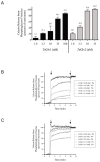
Antimicrobial peptides are generated in insects exposed to pathogens for combating infection. Gloverin is a small cationic antibacterial protein whose expression is induced in the hemocytes and fat body cells of Trichoplusia ni larvae exposed to bacteria. The purpose of this study was to determine the role of gloverin during baculovirus infection. We found that gloverin expression is induced in T. ni systemically infected with the baculovirus Autographa californica M nucleopolyhedrovirus (AcMNPV). Two gloverin genes were cloned using RNA isolated from the hemocytes of T. ni larvae that were systemically infected with AcMNPV budded virus (BV) and C-terminal 6x-His and V5 epitope tags were incorporated to facilitate gloverin isolation, detection and functional studies. The supernatants of Sf9 cells stably transfected with the two gloverin expression plasmids and affinity purified gloverin proteins reduced the quantity of infectious AcMNPV BV as measured in vitro by plaque assay with untransfected Sf9 cells. Nanomolar concentrations of affinity column purified gloverin protein caused calcein to be rapidly released from unilamellar vesicles comprised of phosphatidylglycerol, but not from vesicles made up of phosphatidylcholine, suggesting that gloverin interaction with membranes is rapid and affected by membrane charge. Both the BV inactivation and calcein release activities of gloverin increased with higher concentrations of gloverin. These results demonstrate that gloverin is an antiviral protein that interacts with vesicle membranes to cause the contents to be released.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alarcon-Chaidez FJ, Müller-Doblies UU, Wikel S. Characterization of a recombinant immunomodulatory protein from the salivary glands of Dermacentor andersoni. Parasite immunology. 2003;25(2):69–77. - PubMed
-
- Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. In: Neufeld E, Ginsburg V, editors. Methods in enzymology. Vol. VIII: Complex Carbohydrates. 1966. pp. 115–118.
-
- Axen A, Carlsson A, Engström Å, Bennich H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur J Biochem. 1997;247:614–619. - PubMed
-
- Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem Mol Biol. 2009;39(11):792–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

