The ciliary transition zone: from morphology and molecules to medicine
- PMID: 22401885
- PMCID: PMC3331593
- DOI: 10.1016/j.tcb.2012.02.001
The ciliary transition zone: from morphology and molecules to medicine
Abstract
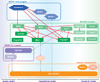
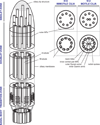
Researchers from various disciplines, including cell and developmental biology, genetics and molecular medicine, have revealed an exceptional diversity of cellular functions that are mediated by cilia-dependent mechanisms. Recent studies have directed our attention to proteins that localize to the ciliary transition zone (TZ), a small evolutionarily conserved subcompartment that is situated between the basal body (BB) and the more distal ciliary axoneme. These reports shed light on the roles of TZ proteins in ciliogenesis, ciliary protein homeostasis and specification of ciliary signaling, and pave the way for understanding their contribution to human ciliopathies. In this review, we describe the interplay of multimeric protein complexes at the TZ, integrating morphological, genetic and proteomic data towards an account of TZ function in ciliary physiology.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella - back to the future! Biology of the cell / under the auspices of the European Cell Biology Organization. 2011;103:249–270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

