Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial
- PMID: 22401913
- PMCID: PMC3412626
- DOI: 10.1016/S1470-2045(12)70075-X
Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial
Abstract
Background: By contrast with many observational studies, women in the Women's Health Initiative (WHI) trial who were randomly allocated to receive oestrogen alone had a lower incidence of invasive breast cancer than did those who received placebo. We aimed to assess the influence of oestrogen use on longer term breast cancer incidence and mortality in extended follow-up of this cohort.
Methods: Between 1993 and 1998, the WHI enrolled 10,739 postmenopausal women from 40 US clinical centres into a randomised, double-masked, placebo-controlled trial. Women aged 50-79 years who had undergone hysterectomy and had expected 3-year survival and mammography clearance were randomly allocated by a computerised, permuted block algorithm, stratified by age group and centre, to receive oral conjugated equine oestrogen (0·625 mg per day; n=5310) or matched placebo (n=5429). The trial intervention was terminated early on Feb 29, 2004, because of an adverse effect on stroke. Follow-up continued until planned termination (March 31, 2005). Consent was sought for extended surveillance from the 9786 living participants in active follow-up, of whom 7645 agreed. Using data from this extended follow-up (to Aug 14, 2009), we assessed long-term effects of oestrogen use on invasive breast cancer incidence, tumour characteristics, and mortality. We used Cox regression models to estimate hazard ratios (HRs) in the intention-to-treat population. This study is registered with ClinicalTrials.gov, number NCT00000611.
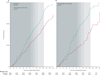
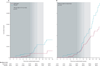
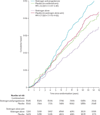
Findings: After a median follow-up of 11·8 years (IQR 9·1-12·9), the use of oestrogen for a median of 5·9 years (2·5-7·3) was associated with lower incidence of invasive breast cancer (151 cases, 0·27% per year) compared with placebo (199 cases, 0·35% per year; HR 0·77, 95% CI 0·62-0·95; p=0·02) with no difference (p=0·76) between intervention phase (0·79, 0·61-1·02) and post-intervention phase effects (0·75, 0·51-1·09). In subgroup analyses, we noted breast cancer risk reduction with oestrogen use was concentrated in women without benign breast disease (p=0·01) or a family history of breast cancer (p=0·02). In the oestrogen group, fewer women died from breast cancer (six deaths, 0·009% per year) compared with controls (16 deaths, 0·024% per year; HR 0·37, 95% CI 0·13-0·91; p=0·03). Fewer women in the oestrogen group died from any cause after a breast cancer diagnosis (30 deaths, 0·046% per year) than did controls (50 deaths, 0·076%; HR 0·62, 95% CI 0·39-0·97; p=0·04).
Interpretation: Our findings provide reassurance for women with hysterectomy seeking relief of climacteric symptoms in terms of the effects of oestrogen use for about 5 years on breast cancer incidence and mortality. However, our data do not support use of oestrogen for breast cancer risk reduction because any noted benefit probably does not apply to populations at increased risk of such cancer.
Funding: US National Heart, Lung, and Blood Institute; Wyeth.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Rowan Chlebowski reported being a consultant for AstraZeneca, Novartis, Amgen, and Pfizer; receiving funding support from Amgen; and serving on speaker’s bureaus for AstraZeneca and Novartis. No other authors reported any other potential conflicts of interest.
Figures

Comment in
-
Oestrogen and breast cancer: results from the WHI trial.Lancet Oncol. 2012 May;13(5):437-8. doi: 10.1016/S1470-2045(12)70110-9. Epub 2012 Mar 7. Lancet Oncol. 2012. PMID: 22401912 No abstract available.
-
Reproductive endocrinology: Estrogen use and breast cancer--an issue resolved?Nat Rev Endocrinol. 2012 Mar 27;8(5):255. doi: 10.1038/nrendo.2012.44. Nat Rev Endocrinol. 2012. PMID: 22450244 No abstract available.
References
-
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. - PubMed
-
- Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. - PubMed
-
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. - PubMed
-
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027–1032. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

