Protein tyrosine nitration of mitochondrial carbamoyl phosphate synthetase 1 and its functional consequences
- PMID: 22402285
- PMCID: PMC3359619
- DOI: 10.1016/j.bbrc.2012.02.114
Protein tyrosine nitration of mitochondrial carbamoyl phosphate synthetase 1 and its functional consequences
Abstract
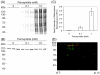
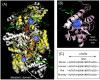
Mitochondria are the primary locus for the generation of reactive nitrogen species including peroxynitrite and subsequent protein tyrosine nitration. Protein tyrosine nitration may have important functional and biological consequences such as alteration of enzyme catalytic activity. In the present study, mouse liver mitochondria were incubated with peroxynitrite, and the mitochondrial proteins were separated by 1D and 2D gel electrophoresis. Nitrotyrosinylated proteins were detected with an anti-nitrotyrosine antibody. One of the major proteins nitrated by peroxynitrite was carbamoyl phosphate synthetase 1 (CPS1) as identified by LC-MS protein analysis and Western blotting. The band intensity of nitration normalized to CPS1 was increased in a peroxynitrite concentration-dependent manner. In addition, CPS1 activity was decreased by treatment with peroxynitrite in a peroxynitrite concentration- and time-dependent manner. The decreased CPS1 activity was not recovered by treatment with reduced glutathione, suggesting that the decrease of the CPS1 activity is due to tyrosine nitration rather than cysteine oxidation. LC-MS analysis of in-gel digested samples, and a Popitam-based modification search located 5 out of 36 tyrosine residues in CPS1 that were nitrated. Taken together with previous findings regarding CPS1 structure and function, homology modeling of mouse CPS1 suggested that nitration at Y1450 in an α-helix of allosteric domain prevents activation of CPS1 by its activator, N-acetyl-l-glutamate. In conclusion, this study demonstrated the tyrosine nitration of CPS1 by peroxynitrite and its functional consequence. Since CPS1 is responsible for ammonia removal in the urea cycle, nitration of CPS1 with attenuated function might be involved in some diseases and drug-induced toxicities associated with mitochondrial dysfunction.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome. Res. 2009;8:3222–3238. - PubMed
-
- Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. - PubMed
-
- Sokolovsky M, Riordan JF, Vallee BL. Conversion of 3-nitrotyrosine to 3-aminotyrosine in peptides and proteins. Biochem. Biophys. Res. Commun. 1967;27:20–25. - PubMed
-
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002;33:1451–1464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

