The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion
- PMID: 22402362
- PMCID: PMC3501346
- DOI: 10.1161/ATVBAHA.112.247668
The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion
Abstract
Objective: Blood-brain barrier (BBB) dysfunction caused by activation of matrix metalloproteinases (MMPs) is a pathological feature in vascular/neurological disease. We describe the mechanisms of BBB dysfunction and neuroinflammation as a result of MMP-3/9 activation and disruption of vascular endothelial growth factor (VEGF)-A/VEGFR-2 interaction, impairing effective angiogenesis.
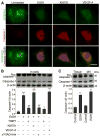
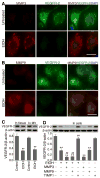
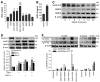
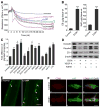
Methods and results: We investigate the hypothesis in human brain endothelial cells and animal model of chronic alcohol ingestion. Proteome array analysis, zymography, immunofluorescence, and Western blotting techniques detected the activation, expression, and levels of MMP-3 and MMP-9. We found that degradation of VEGFR-2 and BBB proteins, for example, occludin, claudin-5, and ZO-1 by MMP-3/9, causes rupture of capillary endothelium and BBB leakiness. Impairment of BBB integrity was demonstrated by increased permeability of dye tracers and Fluo-3/calcein-AM-labeled monocyte adhesion or infiltration and decrease in transendothelial electric resistance. Alcohol-induced degradation of endothelial VEGFR-2 by MMP-3/9 led to a subsequent elevation of cellular/serum VEGF-A level. The decrease in VEGFR-2 with subsequent increase in VEGF-A level led to apoptosis and neuroinflammation via the activation of caspase-1 and IL-1β release. The use of MMPs, VEGFR-2, and caspase-1 inhibitors helped to dissect the underlying mechanisms.
Conclusions: Alcohol-induced MMPs activation is a key mechanism for dysfunction of BBB via degradation of VEGFR-2 protein and activation of caspase-1 or IL-1β release. Targeting VEGF-induced MMP-3/9 activation can be a novel preventive approach to vascular inflammatory disease in alcohol abuse.
Figures

References
-
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. - PubMed
-
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. - PubMed
-
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. - PubMed
-
- Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. - PubMed
-
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

