Metalloproteinase processing of HBEGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids
- PMID: 22402363
- PMCID: PMC3357069
- DOI: 10.1161/ATVBAHA.111.241257
Metalloproteinase processing of HBEGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids
Abstract
Objective: Atherosclerosis is a chronic inflammatory disease initiated by monocyte recruitment and retention in the vessel wall. An important mediator of monocyte endothelial interaction is the chemokine interleukin (IL)-8. The oxidation products of phospholipids, including oxidized 1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine (Ox-PAPC), accumulate in atherosclerotic lesions and strongly induce IL-8 in human aortic endothelial cells (HAECs). The goal of this study was to identify the proximal events leading to induction of IL-8 by Ox-PAPC in vascular endothelial cells.
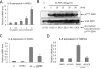
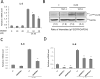
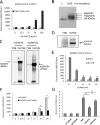
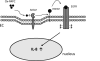
Methods and results: In a systems genetics analysis of HAECs isolated from 96 different human donors, we showed that heparin-binding EGF-like growth factor (HBEGF) transcript levels are strongly correlated to IL-8 induction by Ox-PAPC. The silencing and overexpression of HBEGF in HAECs confirmed the role of HBEGF in regulating IL-8 expression. HBEGF has been shown to be stored in an inactive form and activation is dependent on processing by a dysintegrin and metalloproteinases (ADAM) to a form that can activate the epidermal growth factor (EGF) receptor. Ox-PAPC was shown to rapidly induce HBEGF processing and EGF receptor activation in HAECs. Using siRNA we identified 3 ADAMs that regulate IL-8 induction and directly demonstrated that Ox-PAPC increases ADAM activity in the cells using a substrate cleavage assay. We provide evidence for one mechanism of Ox-PAPC activation of ADAM involving covalent binding of Ox-PAPC to cysteine on ADAM. Free thiol cysteine analogs showed inhibition of IL-8 induction by Ox-PAPC, and both a cysteine analog and a cell surface thiol blocker strongly inhibited ADAM activity induction by Ox-PAPC. Using microarray analyses, we determined that this ADAM pathway may regulate at least 30% of genes induced by Ox-PAPC in HAECs.
Conclusions: This study is the first report demonstrating a role for the ADAM-HBEGF-EGF receptor axis in Ox-PAPC induction of IL-8 in HAECs. These studies highlight a role for specific ADAMs as initiators of Ox-PAPC action and provide evidence for a role of covalent interaction of Ox-PAPC in activation of ADAMs.
Figures


References
-
- Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

